By Michael Cook, Sudhir Ananthachar and Shiying Zheng, Evonik Corporation
In the challenging world of industrial, protective, and marine coatings, two-component (2K) epoxy systems are established as the benchmark technologies due to the combined offerings of excellent corrosion protection and compliance with regional volatile organic compound standards. Today, productivity has emerged as a major driver, and innovation focuses on developing epoxy coatings that have greater application versatility while providing enhanced performance properties, such as dry speed, rapid recoat, and through-cure. For marine and protective coatings, the need is for faster cure and blush-resistant coatings when applied under adverse, low temperatures conditions. In the OEM sector, the wet-on-wet application process means there is a requirement for epoxy systems to provide rapid overcoatability with polyurethane and/or polycarbamide topcoats within minutes after initial application of the primer. In this sector, the drivers are to increase overall productivity with faster application of multiple layers coupled with lower bake temperatures which can provide energy savings. This article will focus on the performance attributes of a novel polycyclic-aliphatic amine and its use in the development of new epoxy curing agents designed to provide benefit in the above markets. The supporting data includes the functional properties thermal analysis, glass transition (Tg ), and infrared (IR)-cure profile, confirming the rapid through-cure and crosslinking capabilities in epoxy systems. In addition, a review of model coating formulations and their key performance attributes, including the rapid recoat times, improved intercoat adhesion, and excellent corrosion protection properties will be discussed.
Introduction
Global megatrends are reshaping the world we live in, driving common requirements of improved productivity and reducing costs, while addressing emerging environmental concerns. Epoxy coatings used in marine and protective coatings are based on either solid or liquid epoxy resins, derived from bisphenol A digylcidylether and cured in combination with polyamides or modified aliphatic or cycloaliphatic amine hardeners. Typical modifications include amine adducts, Mannich bases, phenalkamines, and specialty ketimine curatives,1 designed to ensure an optimum balance of handling and end performance properties. With the introduction of maximum volatile organic compound (VOC) limits in coating applications, development work in epoxy coatings has moved away from using traditional solid epoxy resins (SER) to systems based on the lower viscosity liquid epoxy resin (LER). A standard solvent-free LER has a viscosity of 10,000 mPa.s and is characterized by an epoxy equivalent weight (EEW) ±190 (functionality ±2). The use of LER enables the formulator to achieve higher coating solids (≥80%) vs the traditional solventborne SER systems where solids are in the 40%–60% range. This approach influences the handling and performance characteristics of the formulated coating. Reaction kinetic studies demonstrate that there is a negative impact on the workable pot life coupled with an extension of the dry time. The latter effect is due to the polymeric network having to react and build up sufficient molecular weight to reach the gel point or dry-to-touch state, whereas with the SER systems, these are already high molecular weight polymers and dry-to-touch or lacquer dry is observed as soon as the solvents evaporate from the coating film.2
To overcome the slower dry speed, formulators typically incorporate tertiary (3°) amine accelerators (e.g., tris -2,4,6-dimethylaminomethyl phenol) at ±5% by weight based on active curing agent into the formulation. The acceleration mechanism of the 3° amine is that it polarizes the C–O bond in the epoxy group and makes it more susceptible to nucleophilic attack with the primary (1°) and secondary (2°) amines present in the additional curing agent.3
Formulators can only use small quantities of this type of accelerator because a high concentration can result in driving the homopolymerization of the epoxy resin in favor of the preferred crosslinking reaction between the epoxy resin and amine curing agent. Excessive homopolymerization often results in brittle coatings and free unreacted amines, the presence of which may result in a decrease in the corrosion resistance properties of the cured film.
Our approach to amine design has resulted in the development of a new polycyclic amine, Poly [HCA], that has a balance of 3°, 2°, and 1° amines built into the polymeric backbone.4 Poly [HCA] delivers a unique balance of properties by acting as a reactive accelerator and co-curative all in one. The amine is capable of enhancing the molecular weight build up in applied epoxy so coatings can reach their dry-to-touch state faster, while driving the crosslinking reaction so that coatings achieve rapid through-cure at both ambient and low-temperature cure conditions. This is evident when monitoring the degree of reaction via infrared (IR) spectroscopy. The Poly [HCA] shows a higher level of hydroxyl formation compared to the standard tertiary amine accelerator, which in the presence of liquid epoxy resin is shown to form ether linkages (Figure 1). High ether linkage formation is a clear indicator that the epoxy system undergoes homopolymerization rather than amine-epoxy crosslinking during the curing process.
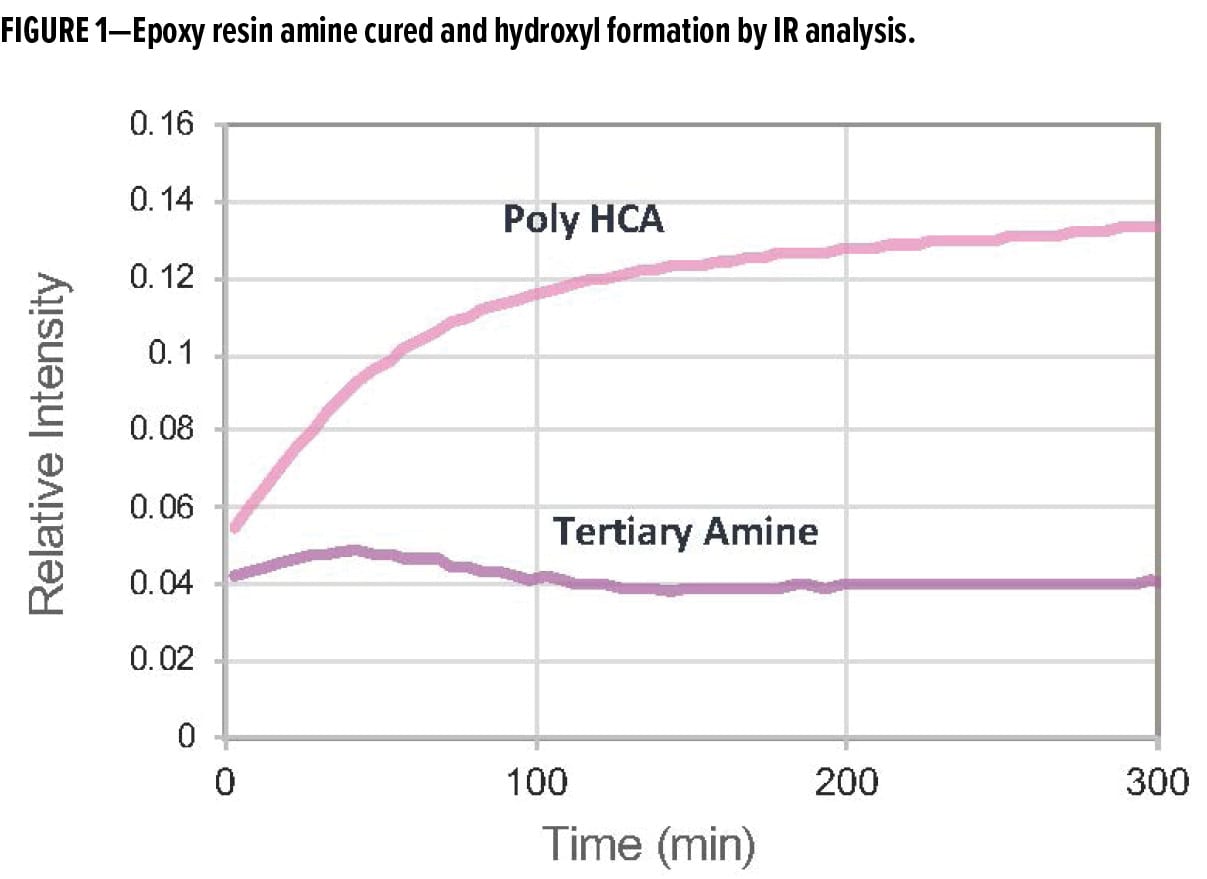
The novel polycyclic amine technology enables rapid property development when formulated with conventional polyamides, modified polyamides, aliphatic, and other formulated epoxy curing agents. Examples of curing agents developed based on Poly [HCA] amine include two new polycyclic amine polyamides, RDPA-1 and RDPA-2. The new polyamides undergo rapid cure, which enables application of topcoats based on similar epoxy or polyisocyanate technology within 15–30 min while maintaining excellent intercoat adhesion and corrosion protection. It provides excellent film appearance without loss of gloss, distinction of image and surface wrinkling, and dive-back of topcoats into the primer. This performance attribute allows applicators to spray-apply multiple layers within quick succession, to increase the overall productivity of the coating application at the job site.
Key Features and Benefits of the Poly [HCA] Amine Building Block
Common amines used in the design of amine curing agents are classified as aliphatic or cycloaliphatic amines, examples of which include diethylenetriamine (DETA), triethylenetetramine (TETA), diaminocyclohexane (DACH), and isophoronediamine (IPD). Although aliphatic amines such as DETA and TETA have high functionality and reactivity, they have a strong tendency to blush and form amine carbamate due to poor compatibility of the amine with the epoxy resin. On the other hand, cycloaliphatic amines have excellent compatibility with epoxy resin because of the cyclocaliphatic backbones but have slower reactivity than aliphatics, especially at low temperature. Good compatibility between curing agent and epoxy resin is essential to provide coatings with good surface appearance, excellent overcoatability, and corrosion resistance. It has been a challenge in epoxy coatings to design a new amine building block that possesses the benefits of both the aliphatic and cycloaliphatic amines: the reactivity of an aliphatic amine and the resin compatibility of a cycloaliphatic amine. As highlighted, Poly [HCA] is a novel polyheterocyclic amine that crosslinks with an epoxy resin delivering fast through-cure while maintaining good resin compatibility over a range of application temperatures. Poly [HCA] is a polymeric amine with moderate viscosity, has a low color (water white), and can be used as a sole curing agent or as a co-curing agent with other amines. The general handling properties are outlined in Table 1, and the following sections detail the amine’s unique performance properties.
![Properties of Poly [HCA] Amine](https://www.paint.org/wp-content/uploads/2019/11/TABLE-1.jpg)
Fast Property Development of Poly [HCA] Amine
Poly [HCA] amine was evaluated against aliphatic amines, cycloaliphatic amines, and Mannich base curing agents to exemplify its fast development of coating properties. For this, Poly [HCA] amine was plasticized with ±30 wt% of benzyl alcohol, comparable to the concentration contained in many commercial curing agents, and cured with standard bisphenol A liquid epoxy resin (EEW=190) at 1:1 stoichiometry. Clear coating formulations for thin film set time (TFST), Persoz hardness, and gloss were deposited on glass substrates at 150 mm wet film thickness using a bird applicator. The TFST was determined using a Beck-Koller recorder, in accordance with ASTM D5895. Persoz hardness was conducted in accordance with ASTM D4366 after coatings were cured at 23°C, 10°C, and 5°C, and 50% RH for designated cure duration of one day, two days, and seven days. Gloss was determined at an angle of 20° and 60° using a Gardner gloss meter according to ASTM D523. Measurements were made with the glass panel placed on a black cardboard background to minimize reflection.
The test summary in Table 2 clearly shows that clear coatings based on Poly [HCA] delivered fast dry speed and Persoz hardness development at ambient and low temperatures. The Poly [HCA] amine demonstrated faster dry speed than a Mannich base curing agent, which is typically used as fast curative especially for low-temperature cure. Poly [HCA] coatings also exhibit good coating appearance similar to cycloaliphatic amine, and better than the Mannich base curing agent. High gloss coating is an indication of good compatibility between resin and curing agents. By comparison, the aliphatic amine exhibited poor compatibility with the epoxy resin and, as such, the resultant coatings were greasy and the Persoz hardness could not be obtained.
In addition, Figure 2 shows the cure and property development of clear coatings across a range of application temperatures, from ambient temperature to low temperature down to 5°C. The fast cure property is demonstrated by the early hardness development and ultimate hardness both at ambient and low application temperatures, thus making the Poly [HCA] amine an ideal amine building block for a variety of coating systems where low temperature cure is critical. Poly [HCA] amine not only delivers fast cure speed, but also shows good compatibility with epoxy resin.
![Cure and property development of Poly [HCA] amine.](https://www.paint.org/wp-content/uploads/2019/11/Evonik-Cook-Figure-2.jpg)
Fundamental Study of Fast Cure Mechanism of Poly [HCA] Amine
To understand the cure mechanism of Poly [HCA] amine, dynamic mechanical analysis (DMA) was utilized to monitor the cure process. DMA provides the mechanical property information and the crosslinking density of the cured samples. The crosslinking density is expressed as the average molecular weight between crosslinking points, Mc, and is calculated from the analysis data, while mechanical property such as storage modulus was measured during the analysis.5 In this experiment, Poly [HCA] amine was compared with a cycloaliphatic amine and an aliphatic amine. The amines were cured with standard bisphenol A liquid epoxy resin at 1:1 stoichiometry, and the samples were prepared by making the plaques in silicone rubber molds and allowed to cure at ambient temperature and humidity for a week.
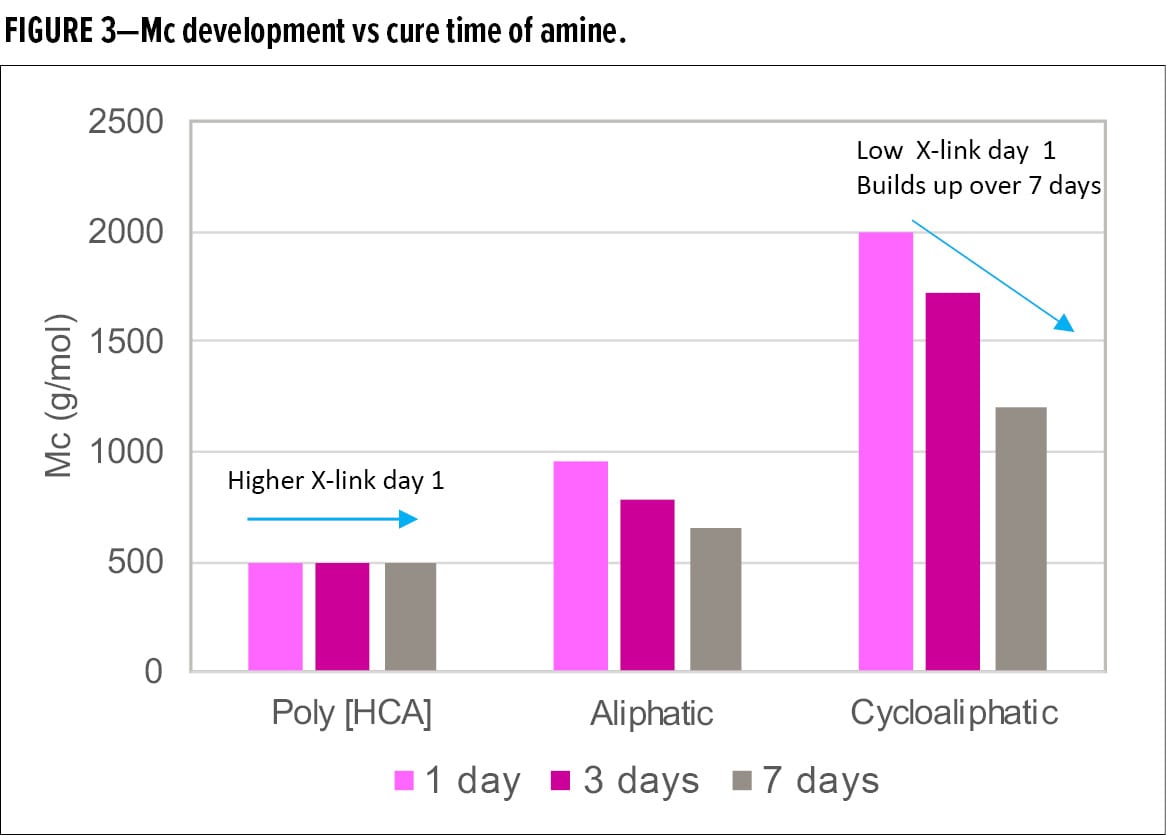
Figure 3 compares the average molecular weight between crosslinking points, Mc, of the samples of the Poly [HCA] amine, an aliphatic amine, and a cycloaliphatic amine after one-day, three-day, and seven-day cure. Mc of Poly [HCA] amine is low and remained unchanged after one-day cure, while Mc of aliphatic amine and cycloaliphatic amine are higher after day 1 and then decrease over time, indicating lower initial degree of crosslinking. However, further crosslinking develops over time, thus a longer reaction time is necessary to achieve full through-cure. Mc of cycloaliphatic amine was the highest, suggesting the slowest reaction rate and lowest crosslinking density.
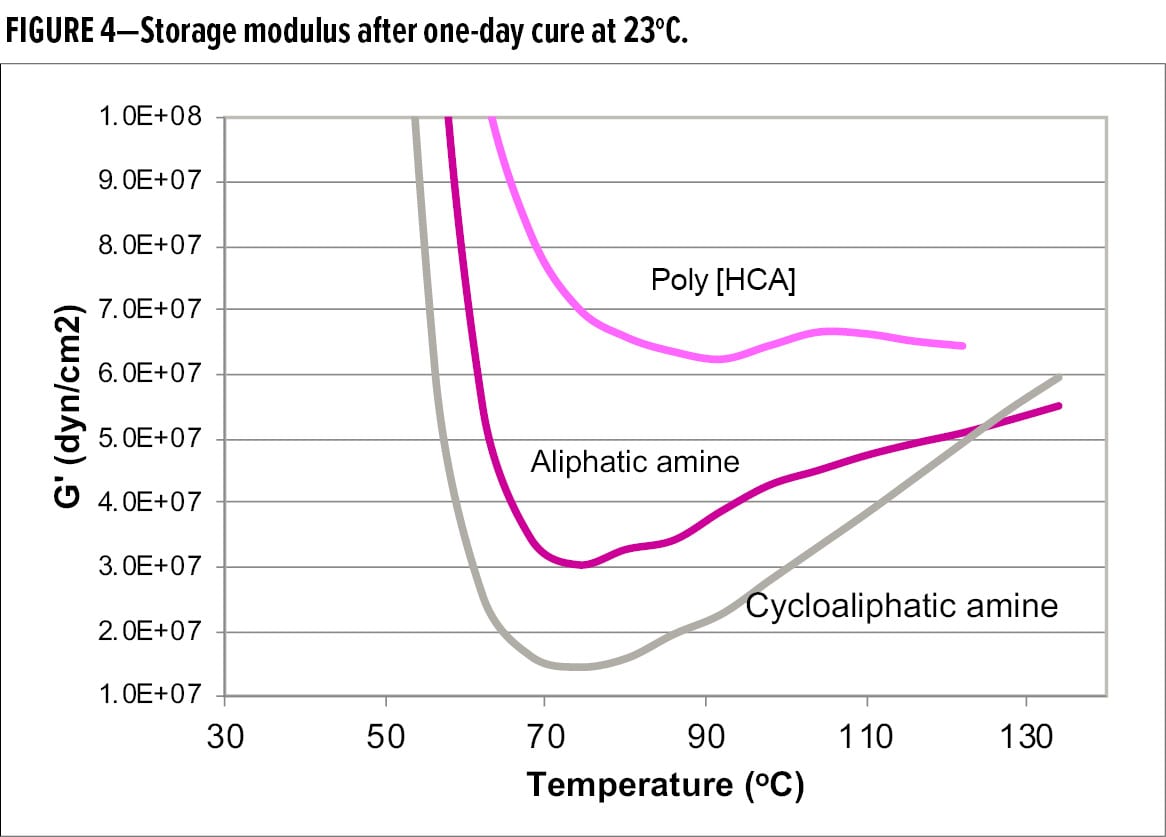
Furthermore, Figure 4 shows the comparison of storage modulus, G’, after one-day cure. G’ of Poly [HCA] remained relatively flat and was the highest among the samples, indicative of highest degree of cure, and no post cure in the rheometer. However, G’ of aliphatic amine and cycloaliphatic amine shows a greater increase, indicating significant post cure in the rheometer. Cycloaliphatic sample exhibited the greatest increase in G’ in the rubbery region, suggesting that it had the lowest degree of cure at ambient temperature. The DMA data of Mc and G’ demonstrate that Poly [HCA] is a much faster curing agent than aliphatic and cycloaliphatic amines, and can reach a high degree of through-cure and crosslinking within a shorter time period.
New Polycyclic Polyamide Curing Agents
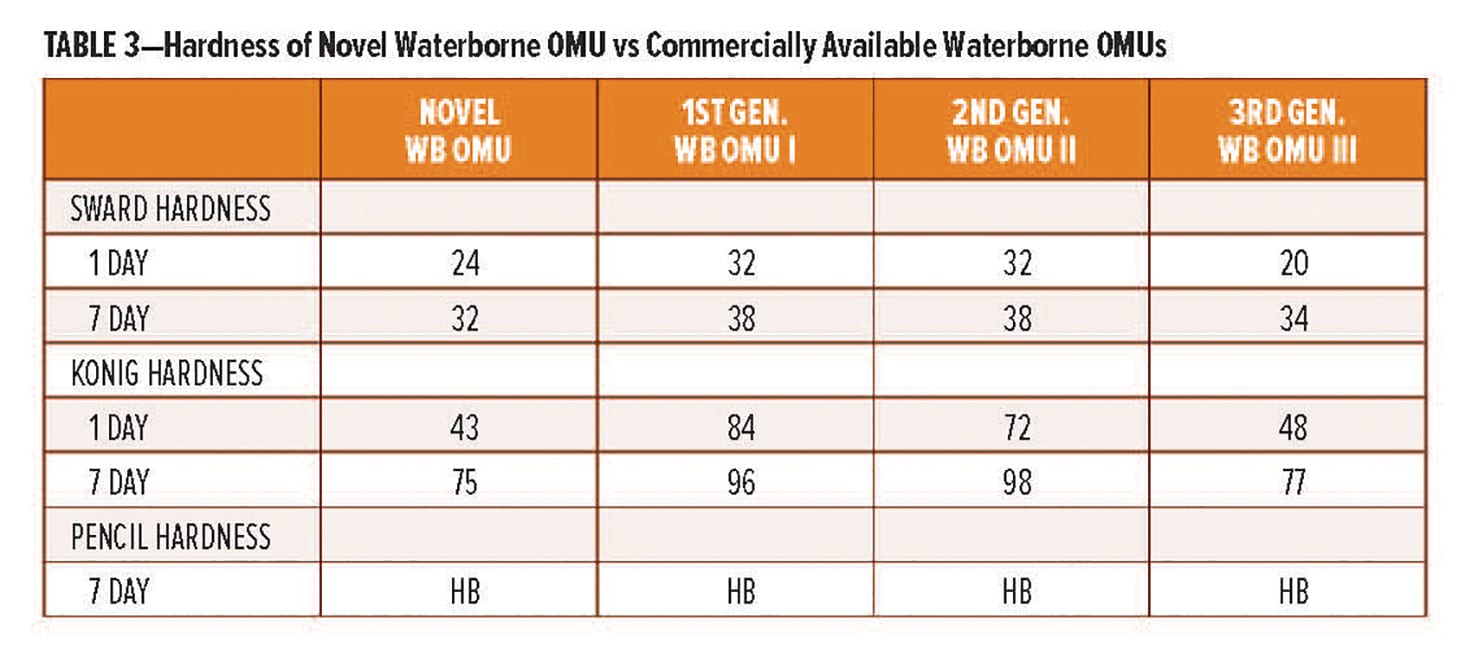
The Poly [HCA] amine is further derivatized to prepare a range of amine curing agents. Of specific interest is the use of the amine is the synthesis of polycyclic-polyamide curing agents that deliver enhanced rapid dry characteristics. Two examples, RDPA-1 and RDPA-2, have been developed and will be reviewed in this article. Both new curing agents provide rapid through-cure at both ambient and low application temperatures, and are capable of being rapidly overcoated either self-on-self or with polyisocyanate-based technologies within a 15 min window after initial application. The technology also delivers the high levels of corrosion resistance properties demanded by the marine, protective, and industrial maintenance coating markets. The typical handling and performance properties of the new curing agents are summarized in Table 3 and subsequent sections below.
Near FTIR spectroscopy (NIR) is a powerful and versatile technique for monitoring transient chemical change during the cure process.6 It offers a unique possibility to obtain detailed information about molecular orientation and relaxation behavior and is an effective tool to monitor the extent of the epoxy-amine reaction. Using an NIR spectrometer, Model 6500, the conversion of oxirane (epoxy) and primary amine during the cure was monitored by the C–O stretch of oxiran ring at 1646 cm-1, and the N–H stretch of the primary amine at 2026 cm-1.
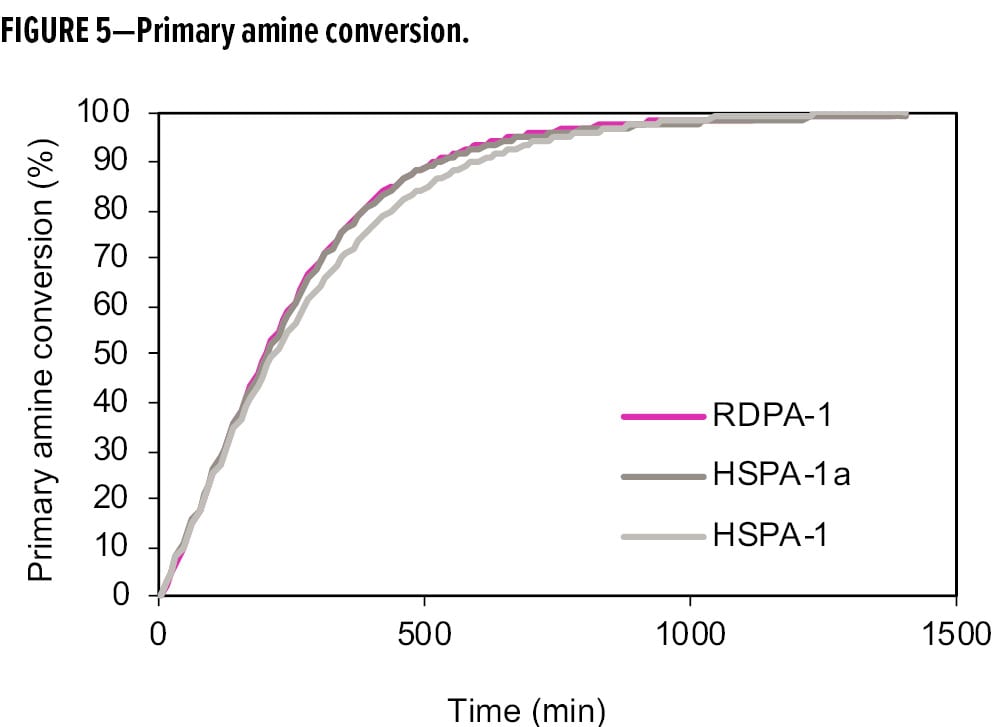
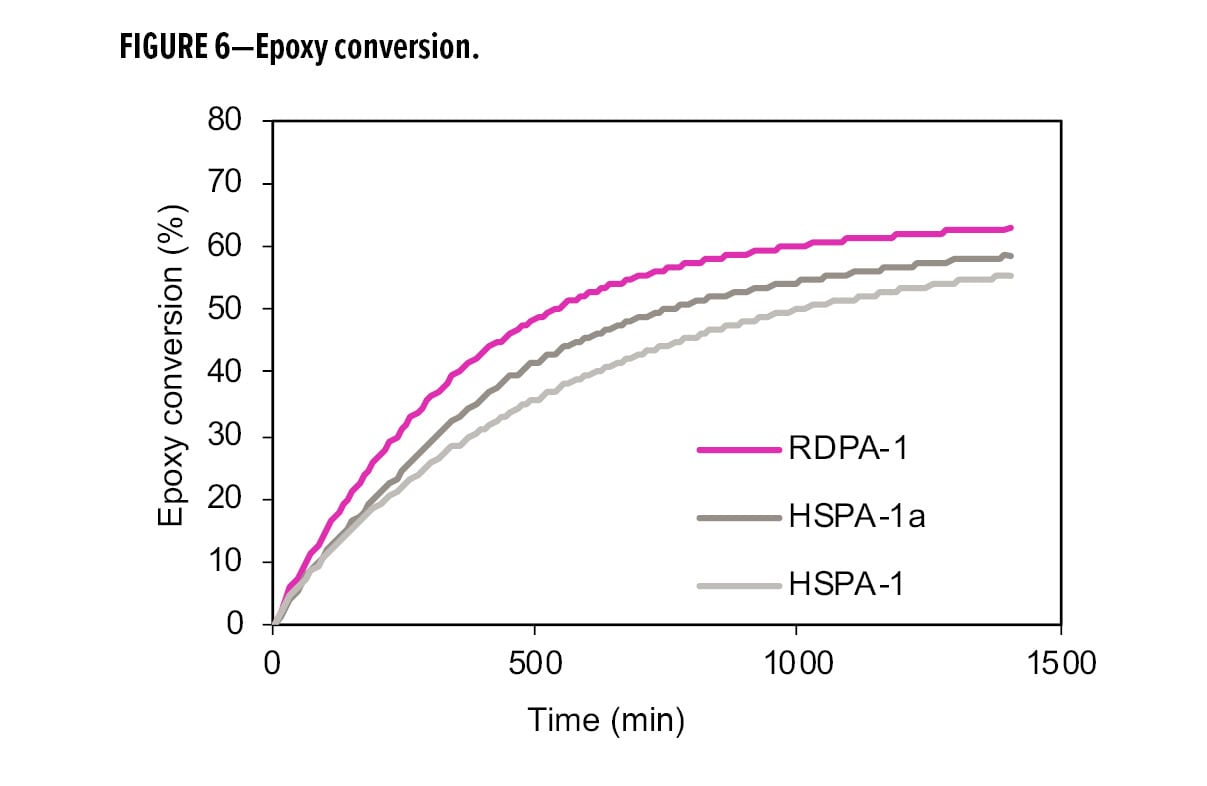
Figures 5 and 6 show the conversion of primary amine and epoxy during the cure process, comparing the RDPA-1 with a standard high solid polyamide (HSPA-1) and the same polyamide blended with a tertiary amine (HSPA-1a). In Figure 5, the primary amine conversions were similar among the three samples due to the fast reactivity of available primary amines with the epoxy. However, in Figure 6, RDPA-1 exhibits the fastest epoxy conversion—faster vs the HSPA-1a and significantly faster vs the unmodified polyamide HSPA-1. Although HSPA-1a showed a fast epoxy consumption vs HSPA-1, there was a lower level of hydroxyl formation in the matrix. The data indicates that a high percentage of the epoxy conversion is a result of the epoxy homopolymerization reaction instead of the amine crosslinking with the epoxy resin.
 The conclusion is further collaborated by dynamic mechanical analysis. Table 4 shows the Tg and Mc of the three polyamide systems. The coating system HSPA-1a containing the tertiary amine accelerator exhibited the highest Tg and lowest Mc, followed by RDPA-1, whereas the standard polyamide HSPA-1 exhibited the lowest Tg and highest Mc values. Both modified polyamides had lower Mc vs HSPA-1, indicating a higher degree of crosslinking. However, in the case of HSPA-1a, the presence of the tertiary amine also drives the competing homopolymerization reaction, which in turn, leads to lower Mc, a higher Tg, and potentially an increase in coating brittleness.
The conclusion is further collaborated by dynamic mechanical analysis. Table 4 shows the Tg and Mc of the three polyamide systems. The coating system HSPA-1a containing the tertiary amine accelerator exhibited the highest Tg and lowest Mc, followed by RDPA-1, whereas the standard polyamide HSPA-1 exhibited the lowest Tg and highest Mc values. Both modified polyamides had lower Mc vs HSPA-1, indicating a higher degree of crosslinking. However, in the case of HSPA-1a, the presence of the tertiary amine also drives the competing homopolymerization reaction, which in turn, leads to lower Mc, a higher Tg, and potentially an increase in coating brittleness.
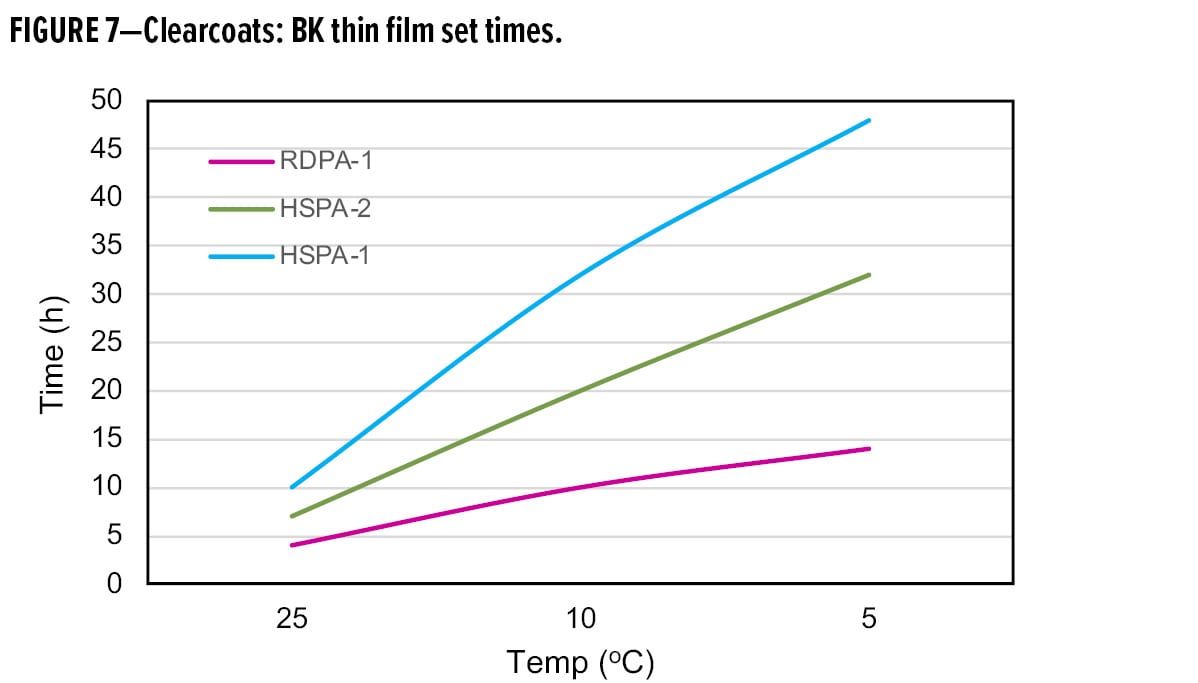
Further evidence supporting the excellent low-temperature cure characteristics of RDPA-1 is shown by the faster dry speed development obtained in clearcoat formulations (Figure 7). When used with liquid epoxy resin, the thin film set times as measured using a Beck Koller (BK) instrument offer a significant improvement over both HSPA-1 and a special modified high solid polyamide adduct (HSPA-2), commonly promoted for low-temperature cure applications. At room temperature, the phase III, thin film set time is 4 h compared with 10 h and 7 h, respectively for HSPA-1 and HSPA-2. At lower application temperatures, the performance benefits of RDPA-1 are clearly demonstrated where a phase III dry time of 14 h is achieved, compared with 48 h for HSPA-1 and 30 h for HSPA-2.
Additional analysis via DSC highlights the differences in the degree of cure development of the polyamides at low application temperatures. Analysis was conducted via measurement of the residual exotherm, during the curing process. Samples were prepared at ambient temperature and then the sealed DSC cells were immediately stored at 5°C in a climate chamber for one to seven days. After the allotted cure time, samples were removed and scanned by DSC (TA Instruments–model Q200) at a ramp rate of 10°C/min. The percentage cure for each sample was calculated by using the following equation.7
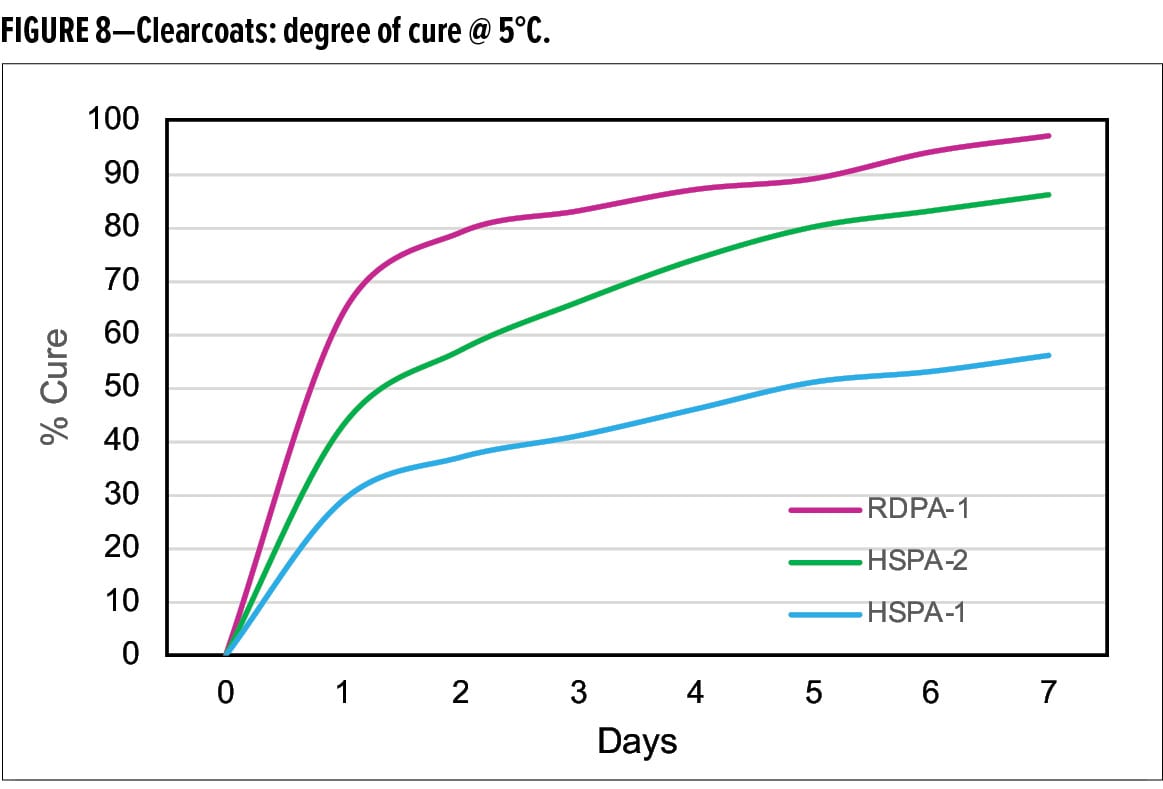
When cured with the standard liquid epoxy resin, DSC analysis shows that the new curing agent RDPA-1 undergoes excellent cure development at 5°C (Figure 8). When compared with HSPA-1 and HSPA-2, RDPA-1 achieves a degree of cure after one day of 64%, which is two times faster than HSPA-1 which only achieved 30% conversion. HSPA-2 achieved 40% degree of cure, however, still significantly slower vs the new curing agent technology. After seven days’ cure at 5°C, the extent of cure for RDPA-1 was >95%, compared with 55% and 83% for HSPA-1 and HSPA-2 respectively.
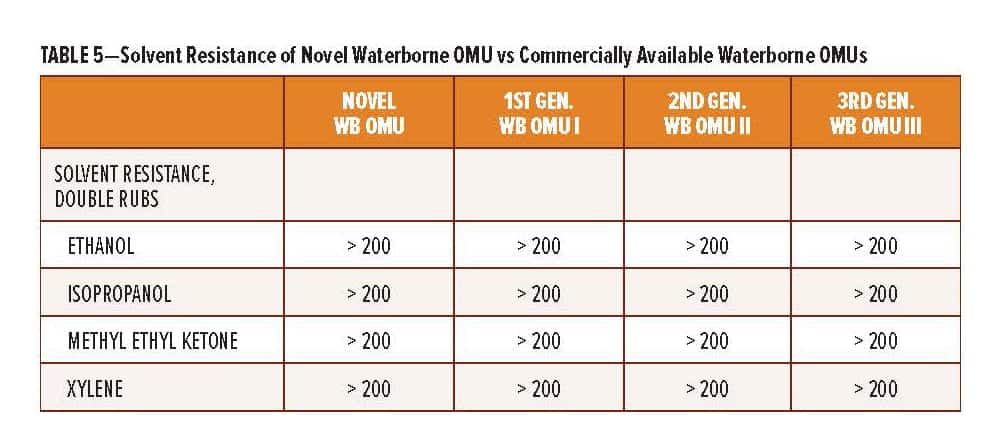
The RDPA-1 curing agent offers additional performance benefits vs existing high solid polyamides used for protective coating applications. The benefits are summarized in Table 5 and include a lower initial curing agent viscosity, thus allowing for high solid coatings where VOC <250 g/L can be achieved. More importantly, the surface appearance and early film integrity, as determined by the ability of the coating to withstand attack from solvents at lower application temperature, is significantly improved.
With RDPA-1, the cured coating is smooth and glossy when systems are applied with a minimum induction time of 5 min. With HSPA-1, film appearance is hazy, and an inherent tackiness is present. MEK resistance using the double rub methodology was employed as a way of assessing early through-cure and solvent resistance. In the case of RDPA-1, clearcoats provided >200 MEK rubs with no down glossing after three days at 5°C, whereas the polyamide systems HSPA-1 and HSPA-2 exhibited no resistance to MEK after one day and after three days the coatings were destroyed, exhibiting < 100 double rubs.
Novel Polyamide Curing Agents for Wet-on-Wet Applications
For OEM applications, there is a growing demand for 2K systems to be applied wet-on-wet to enhance throughput and reduce overall application time and costs. The incumbent technology utilized in sectors where rapid development of cure properties is essential is typically based on Mannich base (phenalkamine type) curing agents that are derived from low molecular weight ethylene diamines. This technology while providing fast dry, often results in coatings with amine blush. This subsequently interacts with the polyisocyanate topcoats, causing surface defects such as down glossing and excessive wrinkling, as shown in Figure 9A. Utilizing the polycyclic amine Poly [HCA], the new rapid cure polyamide, RDPA-2 curing agent is shown to eliminate this phenomenon. When formulated with LER, the surface appearance of the epoxy primer based on RDPA-2 is blush free. This was confirmed via both a visual inspection as well as surface analysis adding a drop of 1% aqueous solution of phenolphthalein indicator to the coating and conducting a swab test.
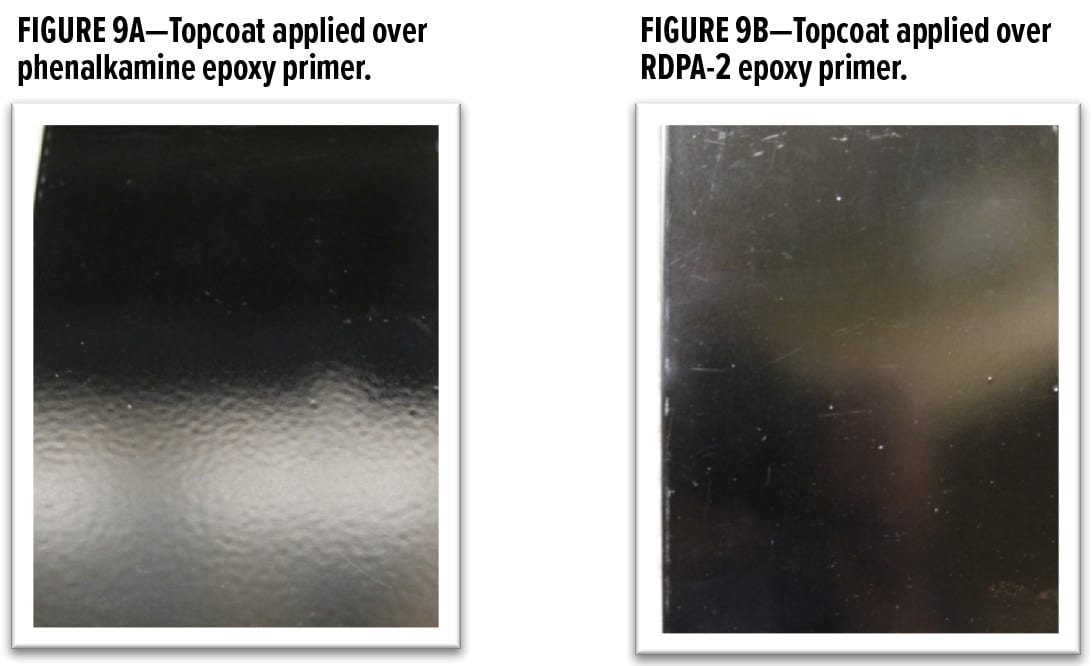
Upon application of a polyurethane topcoat following an initial cure time of 30 min for the base epoxy primer, the topcoat based on a solvent containing polycarbamide dries wrinkle free and with a high surface gloss, as shown in Figure 9B.
For coatings to be used in wet-on-wet applications, systems must build up sufficient coating integrity, which requires coatings to be touch dry or be resistant to dust pickup as quickly as possible after spray application of the primer coating. To demonstrate the performance value of the new polyamide technology, anticorrosive primers were applied on Bondrite B-952 panels at a wet film thickness of 180 µm. Cure properties were evaluated using the thumb twist method, which is an objective test method. In this method, the operator places a thumb on the coating and twists the thumb 90o. If no finger print was observed, the coating was deemed as the minimum dry time required for over coating with the next layer. For this study, the properties of RDPA-1 and RDPA-2 were compared to three alternative technologies. The LOPA-1 polyamide represents a polyamide with an established track record in the coatings industry where it provides an extended recoat window greater than six months.8 HSPA-1 was used as the generic high solids polyamide, and a commercial system (CS-1) was used within the OEM market, based on an accelerated polyamide. The results of the dry film tests are summarized in Table 6.
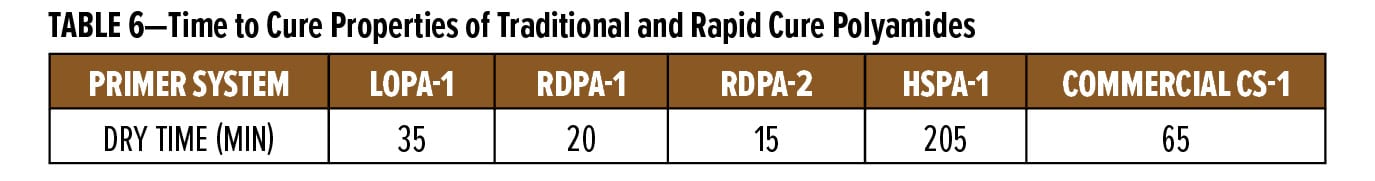
These results clearly demonstrate the faster surface dry characteristics inherent in RDPA-1 and RDPA-2. Both technologies reach the rapid surface dry state significantly faster compared to a standard polyamide, but also faster vs the commercial system CS-1. AlthoughLOPA-1 was faster vs CS-1, this product contains solvent and as such makes it difficult for formulators to meet the need for lower VOC systems. When comparing the reaction kinetics using infrared spectroscopy, RDPA-2 shows a faster conversion of the available primary amine content and higher molecular weight buildup vs comparative samples used in this study.
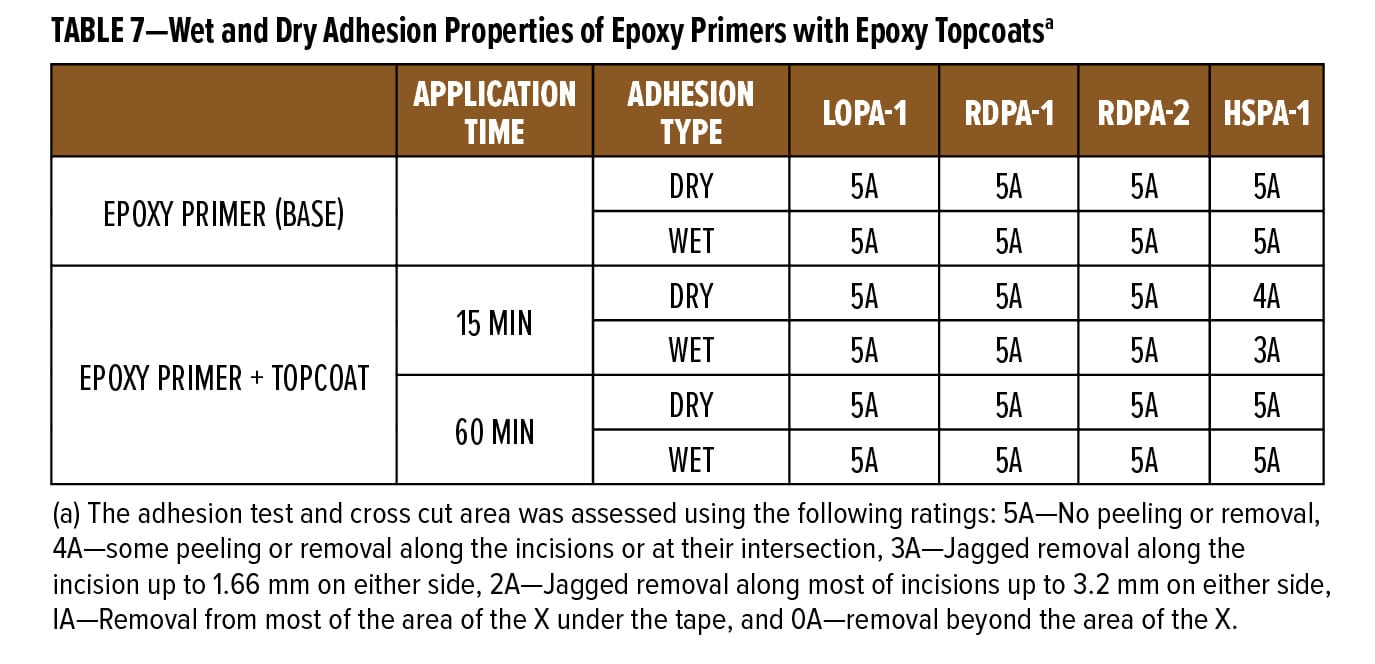
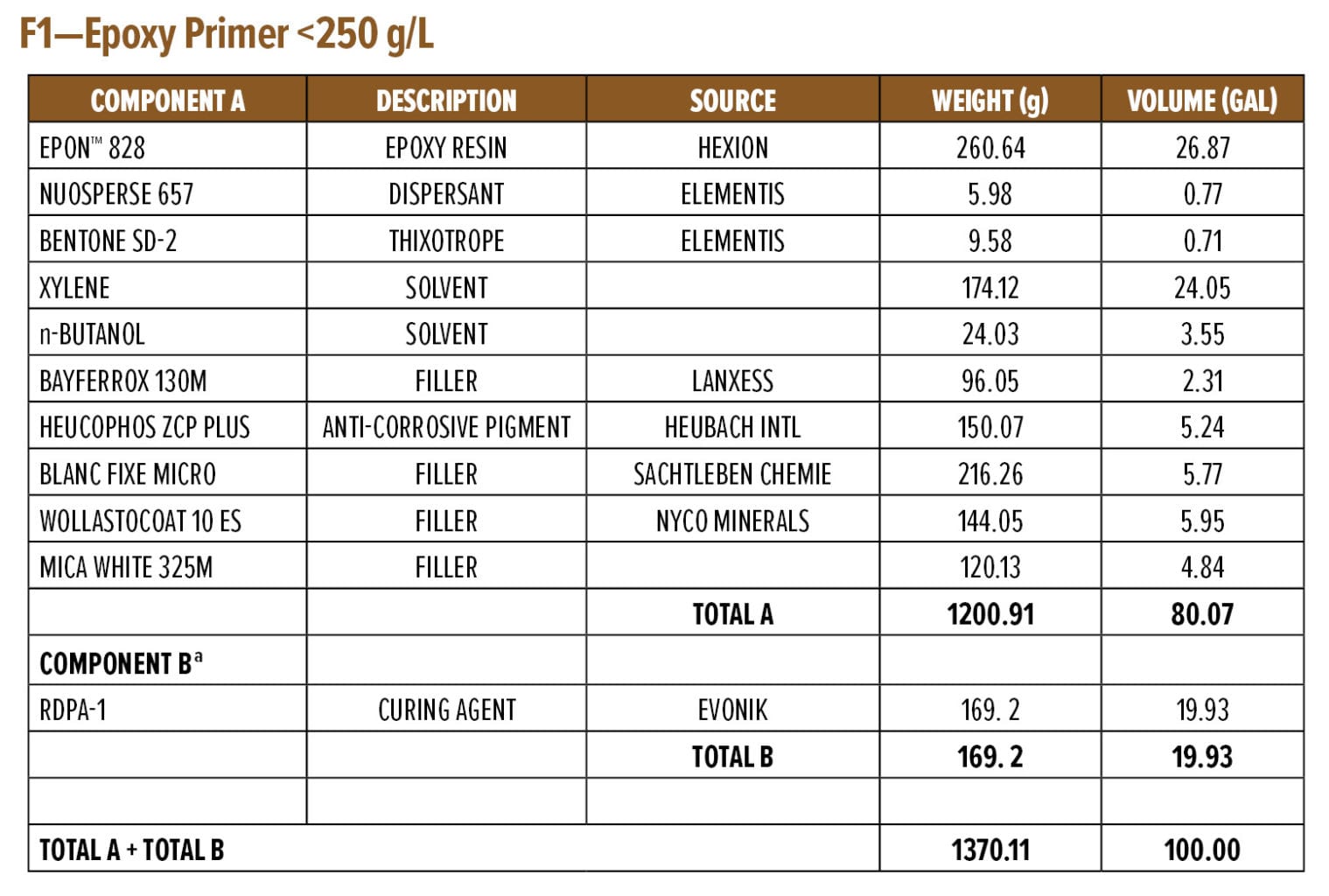
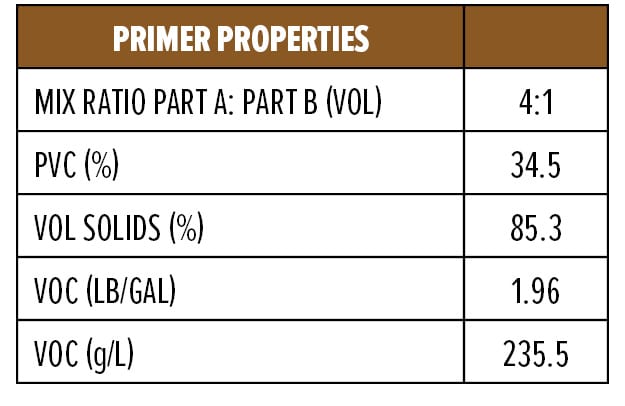
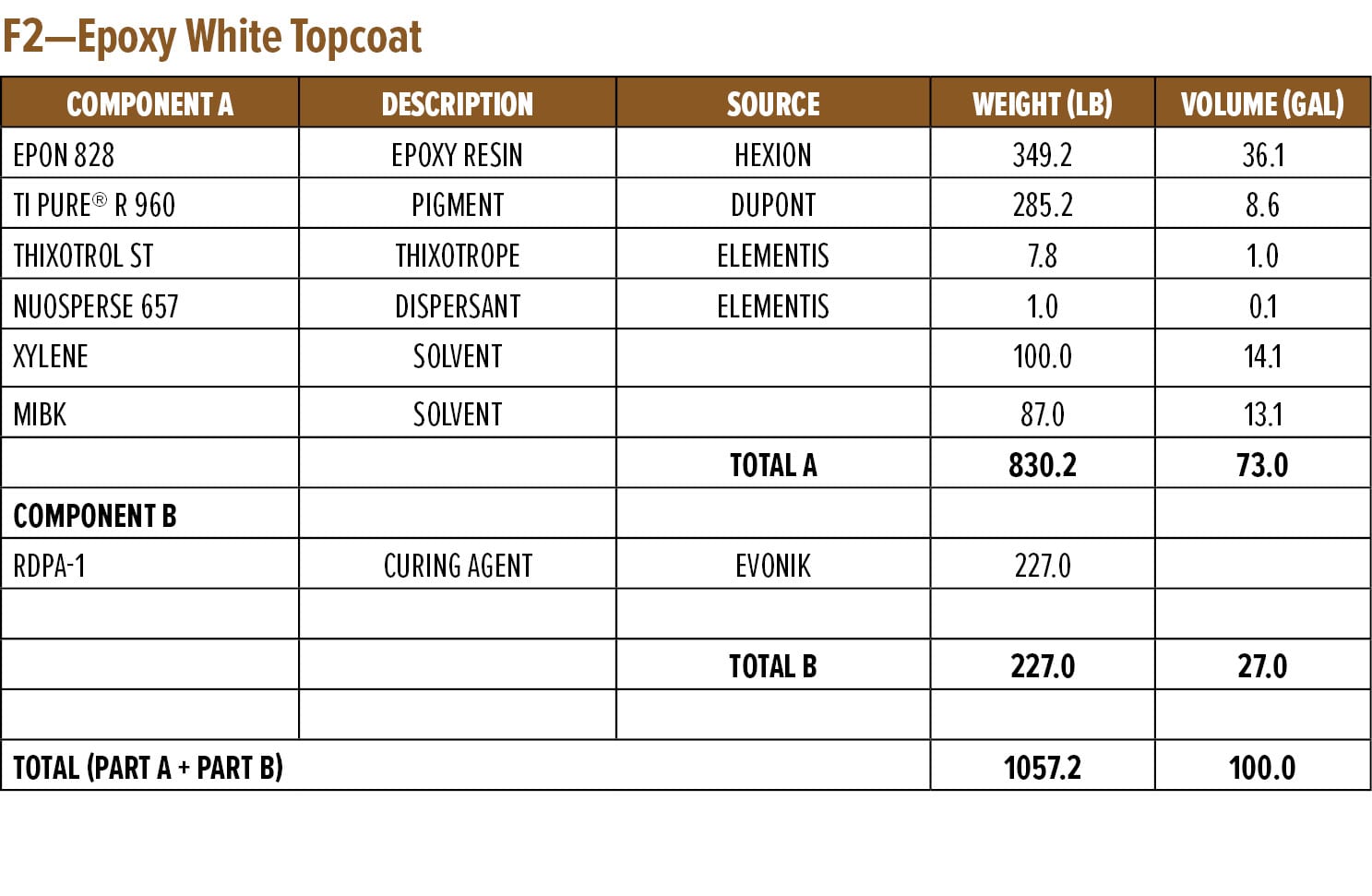
The dry and wet adhesion properties of the epoxy coatings were also evaluated. Tests were conducted on Bondrite B-952 steel panels by applying primers at a wet film thickness of 75 µm followed by a second epoxy white topcoat at 15 min and 60 min intervals. Examples of the primer (F1) and topcoat (F2) are provided in Appendix A. While examples are based on RDPA-1, the same Component A was used for all the curing agents tested, with Component B adjusted for loading based on the AHEW of the corresponding curing agent.
Following seven days cure at ambient temperature, intercoat adhesion was measured as follows: For the wet adhesion test, the panels were immersed in water for additional 24 h. After the immersion time, panels were wiped dry with a paper towel and tested in accordance with ASTM D3359 method A. The results are listed in Table 7.
Rapid Recoat Study Using Polycarbamides
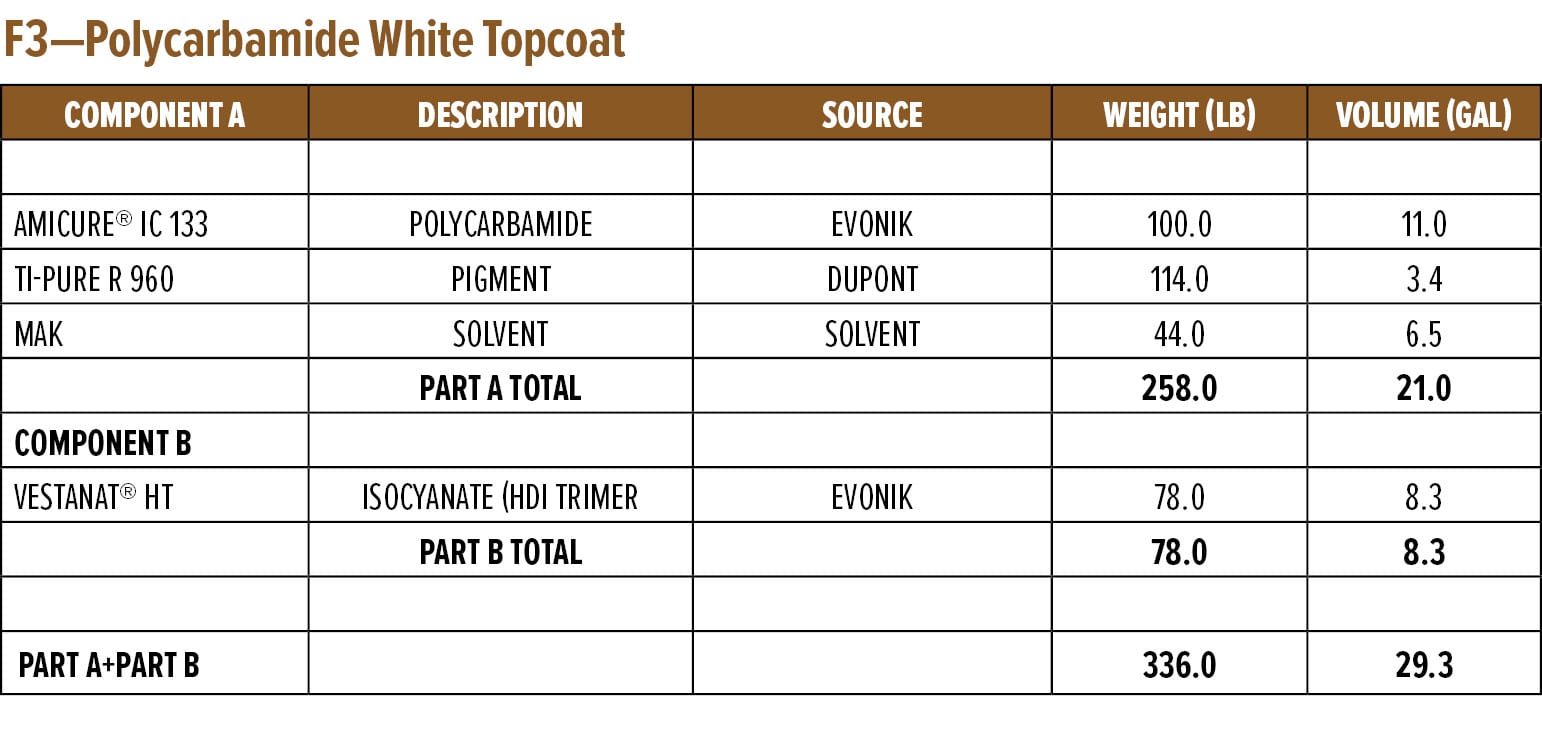
The above study highlighted the excellent rapid dry and overcoatability of RDPA-1 and RDPA-2 with a standard epoxy topcoat. As discussed in the next section, epoxy primers were evaluated for their ability to be rapidly overcoated with a fast cure isocyanate-based system. For this example, a polycarbamide9 (polyaspartic) topcoat based on HDI trimer and a cycloaliphatic diethyl maleate ester-curing agent was used. The rapid recoat properties of RDPA-1 polyamide are compared with that of conventional polyamide HSPA-1 by applying 75 mm of formulated epoxy primer as shown in Appendix A (F1) on Bondrite B-952 panels and cured for 15 min and after 60 min on separate panels. Formulated polycarbamide topcoat Appendix A (F3) was applied (150 mm) after 15 min and 60 min on primed Bondrite panels. The panels were cured for 24 h, and the cross-hatch adhesion test as per ASTM D3359 was conducted. The results are given in Table 8, and the appearance following the dry adhesion tests are depicted in Figures 10 and 11.
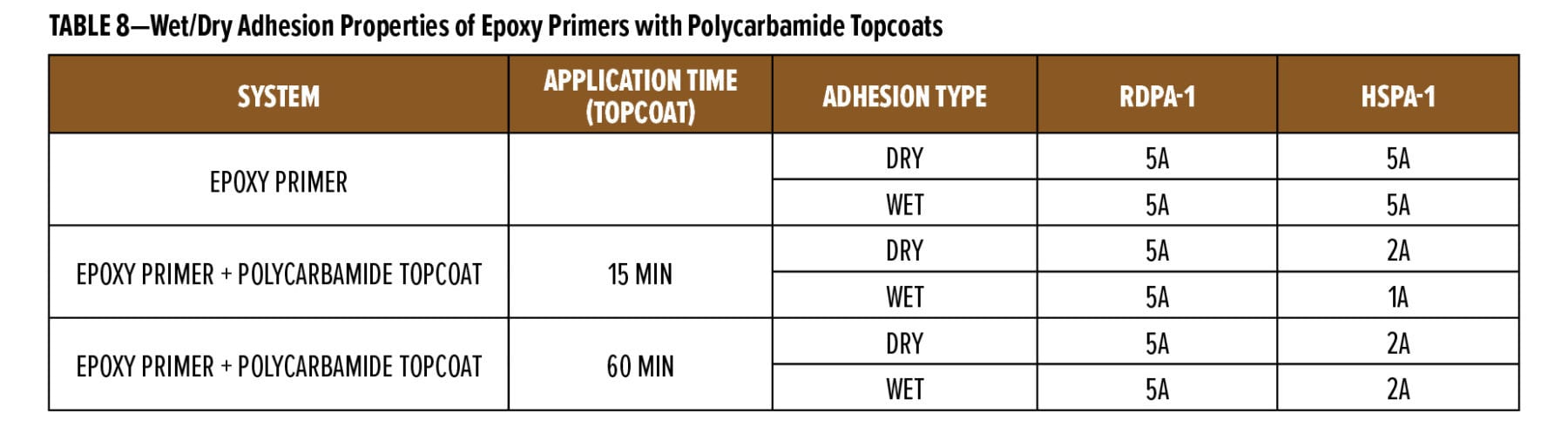
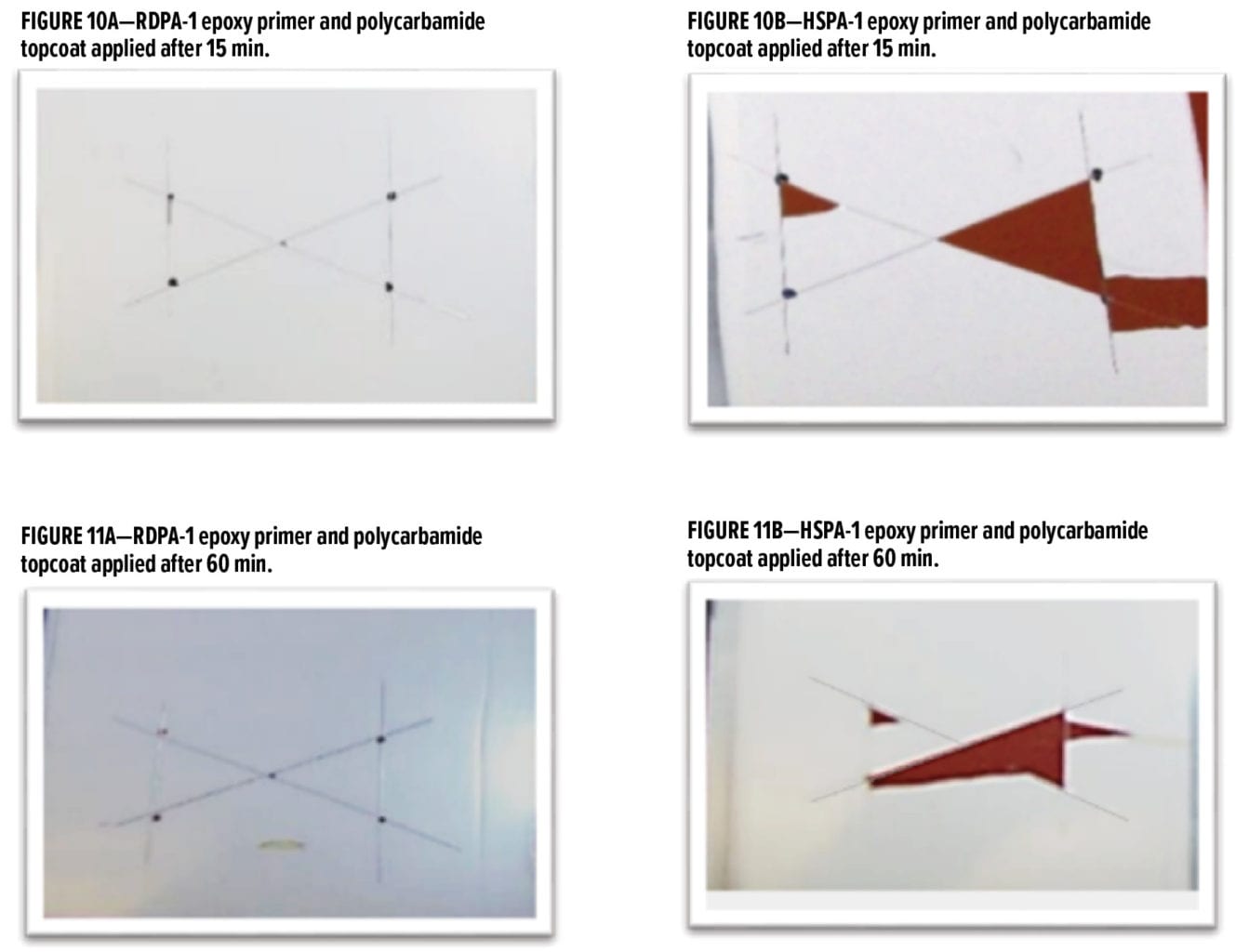
With the modified polyamide RDPA-1, the epoxy primer is dry to touch (finger press) after 15 min cure at 23°C. After application of the polycarbamide topcoat, the surface appearance is smooth and wrinkle free, and high gloss is obtained. The intercoat adhesion results are excellent with a rating of 5A being achieved. With the HSPA-1 curing agent, the epoxy primer is still wet to touch after 15 min and the applied topcoat showed both base primer bleed through and a down glossing after 24 h cure. With this system, the polycarbamide topcoat exhibited very poor intercoat adhesion with a significant surface area delaminating following application and removal of the adhesive tape during the cross-hatch test. When base primers were subjected to a 60-min cure at 23°C followed by application of the polycarbamide topcoat, again excellent surface appearance and adhesion was observed for the RDPA-1 curing agent. There was some improvement with the HSPA-1, with no primer bleed through and an improvement in surface gloss; however, the dry/wet adhesion of the polycarbamide coating was poor with a rating of 2A only. With HSPA-1, the epoxy primer requires approximately 2 h cure10 prior to application of the topcoat to provide the intercoat adhesion performance exhibited by RDPA-1.
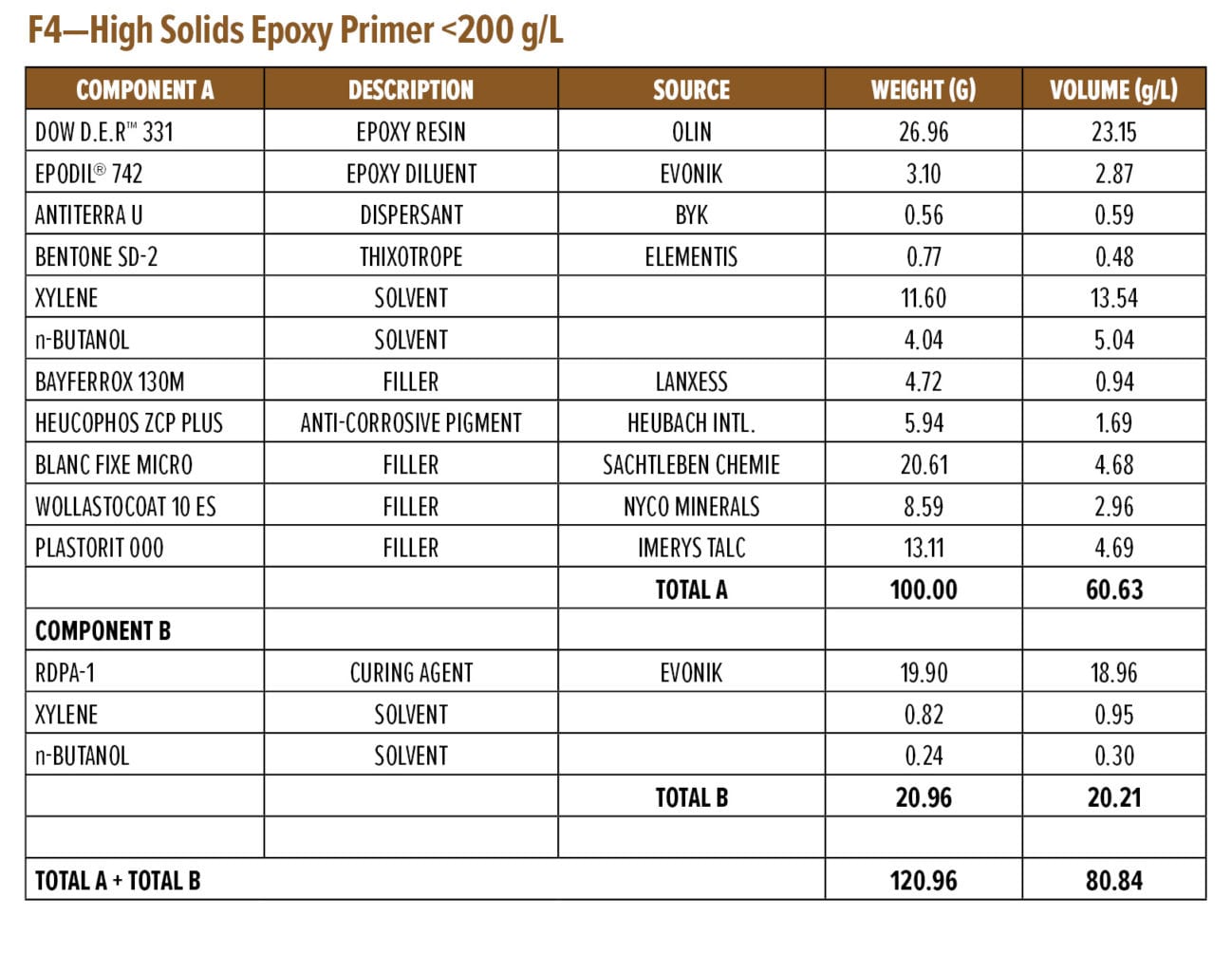
Anti-corrosive Primer Formulation
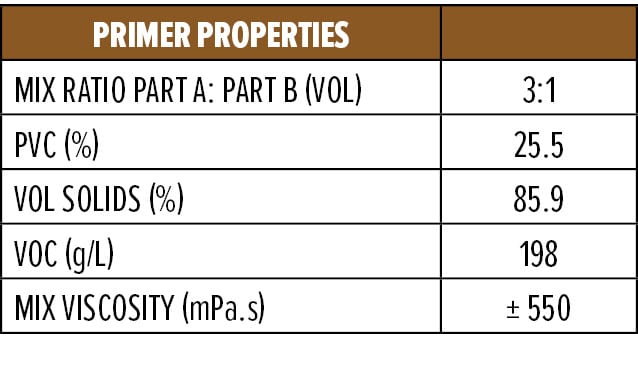
Appendix A (F4) contains a starting point formulation based on RDPA-1 for an anti-corrosive primer. The formulation is a red iron oxide primer based on a modified zinc calcium polyphosphate and offers 86% volume solids at VOC of 198 g/L. Further, the primer formulation has a low mix viscosity of approximately 550 mPa.s, with a pot life of 1.5 h. The primer can be spray applied with conventional spray equipment following 5–10 min of mixing or brush applied to a steel substrate without the addition of extra solvents. The BK phase III dry time is achieved after 2 h, while a dry-to-handle time (thumb twist) is obtained within 8 h.
Accelerated Corrosion Resistance
The anti-corrosive primer formulation based on RDPA-1 was applied to grit blasted (SA 2.5), hot-rolled steel substrate panels. Using conventional spray equipment in double coats, a 180–220 mm dry film thickness resulted. Panels were left to cure at ambient temperature for 10 days prior to testing in salt spray. Panels were scribed and evaluated for field blisters using ASTM B117. Evaluation of scribe creep was rated in accordance with ASTM D1654. At 1000 h intervals, one set of duplicate panels was removed from the test cabinet and evaluated for blistering and rusting. After the visual evaluation was completed, the scribe areas were scraped to expose the underlying metal substrate, allowing for accurate scribe creep measurements. Results for the 2000 h exposure are shown in Figure 12 and reported in Table 9.

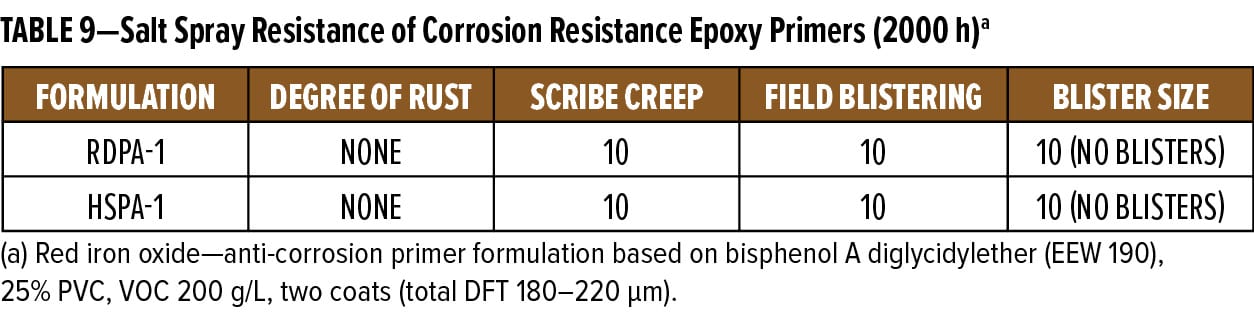
All ratings and scribe creep values reported are the average of the two test panels in each set. The results show that throughout the 2000 h exposure period, the RDPA-1 epoxy primer had similar resistance to blistering and rusting as the standard epoxy-polyamide control coating based on HSPA-1. During the test, no blistering or field rusting was observed on the test panels, thus demonstrating RDPA-1 delivers excellent corrosion resistance properties.
Cathodic Disbondment
The experimental setup is shown in Figure 13. Each test panel is fitted with one intentional holiday of 3 mm diameter in the paint layer, and then covered with a glass cylinder (inside diameter = 99 mm; high 155 mm) on the painted side. The glass cylinders are placed so that from every panel the intentional holiday is situated in the middle of the test area. The glass cylinder is filled with about 1000 mL of electrolyte (artificial seawater). To establish the electrical circuit, a connection to the steel panel is made with a platinum or graphite electrode that is placed in the center of the tank containing the electrolyte. This acts as the anode and is connected to the positive lead from the power supply (Top Hex Cathodic Disbondment Tester). The bare steel of the panel (cathode) is connected with copper wire to the negative lead of the power supply. A reference electrode (saturated calomel) is placed in the test tank for measuring a continuous potential of 1.5 V. After 28 days at room temperature (23°C), the test was stopped. The exposed coatings were checked for loss of adhesion, blistering (ASTM D714) and other defects (discoloration, cracking, etc.). Loss of adhesion was determined by cutting eight radial pies, extending 3 cm from the center of the intentional holiday, by using a sharp-bladed knife. Starting at the intentional holiday and working outward, the degree of disbondment was measured. The disbondment cell and panels after 28 days’ exposure are shown in Figure 13.
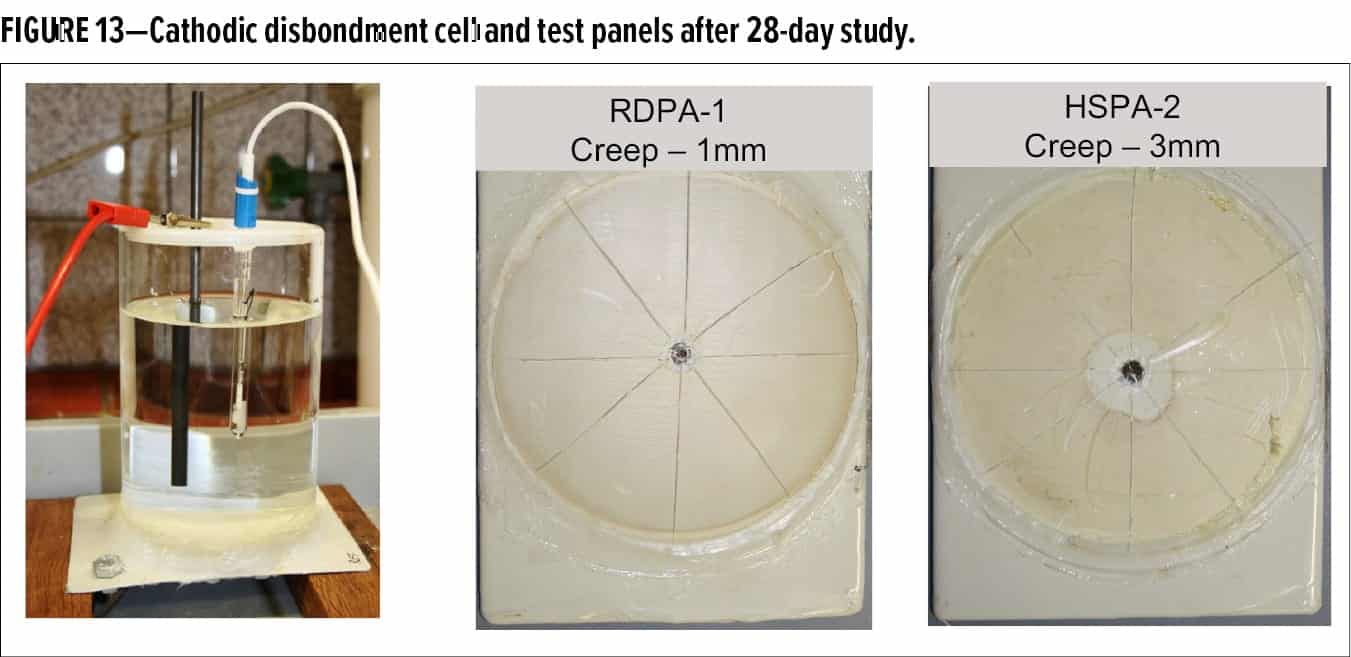
Coatings formulated with RDPA-1 and the benchmark HSPA-2 resulted in an average radial creep of 1 mm and 3 mm respectively. This is well within the requirements of the test and, as such, both coating systems are fit for purpose and meet the ASTM G8-96 standards for pipe coatings requiring excellent cathodic disbondment resistance.
Conclusions
In this article, the utility of a new polycyclic amine, Poly [HCA] that acts as both accelerator and crosslinker for epoxy coatings has been explored. It was demonstrated that the amine allows for a rapid conversion of both epoxy and primary amine functionalities within a 2K epoxy coating system. Thus, coatings achieve a high degree of conversion compared with other aliphatic and cycloaliphatic amines. The buildup of crosslink density is driven by the amine-epoxy reaction compared to the epoxy homopolymerization reaction that is the dominant mechanism observed when conventional tertiary amine accelerators are employed. The new amine technology has further enabled the development of advanced polyamide epoxy curing agents that now exhibit an excellent balance of low-temperature cure, while maintaining the excellent adhesion and corrosion resistance properties required for long-term corrosion protection. In addition, the ambient cure properties of the new curing agents are such that they allow for the development of epoxy primers that exhibit ultra-fast, tack-free set times. The fast set-to-touch combined with a blush-free appearance and excellent early solvent resistance enables the epoxy basecoats to be topcoated with a range of different coating technologies within a very short application window. This property makes the technology ideal for use in wet-on-wet applications for automotive and industrial maintenance applications, where faster technology can provide faster throughput, increasing productivity in the field projects and reducing system and application costs while providing long-term asset protection.
Acknowledgment
The authors would like to thank Evonik colleagues Mike Oberlander, Tom Corby, Aziz Gaffar, and Marcel Peters for carrying out the experimental work, as well as Daniel Totev, Wei Cao, Rob Rasing, and Marcelo Rufo for their technical input.
References
1. Lee, H. and Neville, K., Handbook of Epoxy Resins, McGraw-Hill: New York, NY, 1967.
2. Walker, F.W., Cook, M., Vedage, G., and Rasing, R.,
J. Coat. Technol. Res., 6 (3), 283-313, (2009).
3. Rozenberg, B.A., Adv. Polym. Sci., 75, 113 (1985).
4. Zheng, S., et al., European Patent publication, 3170849A1 (2017).
5. Vratsanos, M., “Rheological and Thermal Characterization of Polymer Coatings,” CoatingsTech, 7, 28-38 (2017).
6. (a) Xu, L., Fu, J.H., and Schlup, J.R.J., Am. Chem. Soc.,116, 2821-2826 (1994); (b) Fu, J. H. and Schlup, J.R.J., Appl. Polym. Sci., 49, 219-227 (1993).
7. Weinmann, D.J., Dangayach, K., and Smith, C.,”Amine-Functional Curatives for Low Temperature Cure Epoxy Coatings,” J. Coat. Technol., 68 (863), 29-37 (1996).
8. Cook, M. and Rasing, R., 19th SLF Congress, 2009.
9. Amicure® IC-133–Evonik Materials.
10. Evonik Materials; unpublished results.
Appendix A—Start Point Formulations
CoatingsTech | Vol. 16, No. 3 | March 2019
