By Stephen Raper, Sara Harris, and Doug Corrigan, ChemQuest Technology Institute
Kyle Kim and Christian Lenges, DuPont Nutrition & Biosciences
INTRODUCTION
Advances in the performance of formulated products through material innovation continue to drive innovation and growth. At the same time, it is becoming increasingly paramount that new materials are also sourced from ideally renewable and overall more sustainable feedstocks using benign processes to meet criteria required within a circular economy context.
Progress in architectural and industrial coatings has focused on providing not only improved paint performance but also optimizing aspects such as pigment efficiency (e.g., reduction of TiO2) and effective gloss management, while maintaining key characteristics such as abrasion performance and overall coating properties. At the same time, continued emphasis has been placed on reducing the environmental footprint through lower volatile organic content (VOC) in paint systems. In addition, increasing efforts have been directed to eventually replace typical petroleum-derived building blocks in coatings formulations with more sustainable, potentially renewable material alternatives.
However, the transition to performance-advantaged renewable building blocks, which are accessible at an enabling cost position and are also based on fungible, readily available raw materials produced in a sustainable and scalable industrial process, remains challenging across material industries. This article discusses one specific example of renewable based additive technology to meet the stated industry performance needs and objectives.
Engineered Polysaccharide Fundamental Material Properties
Structurally, polysaccharides are highly diverse biopolymers composed of repeating glucose units linked via glycoside bonds. The range of polysaccharide structural characteristics, such as linkage isomers, the degree of polymerization, chain branching, and aggregation through strong intermolecular hydrogen bonding, allows these materials to be found in nature as structure-forming, essentially insoluble, highly aggregated materials (e.g., cellulose) or as water-soluble thickening materials with often various biological functions (e.g., starches, various gums).
The interaction of individual polysaccharide polymer chains through hydrogen bonding to form associated supramolecular structures allows for deliberate material engineering from the nano to micron scale. For example, extensive works on various process options to access and control submicron-scale cellulose-based materials (i.e., micro-/nano-fibrillated cellulose, micro-/nano-cellulose) are reported.1,2 Enzymatic polymerization allows for a novel, controlled path towards engineering of nano- to micron-scale primary structures within aggregated, typically micron-sized polysaccharide materials. Efficient and scalable methods to apply a monomer-based (e.g., sucrose), controlled polymerization protocol using an enzyme catalyst to engineer polysaccharide materials is emerging for first commercial, large-scale applications.
The specific family of biocatalysts is selected from the general class of glucosyltransferase (GTF) enzymes, and the polymers can be produced by reacting a solution of sucrose with this GTF enzyme. For the work described here, the alpha-1,3-glucan utilized has a typical degree of polymerization of 800 glucose repeat units with a polydispersity in the range of 1.7–2.0, as controlled by the polymerization process conditions (Figure 1).
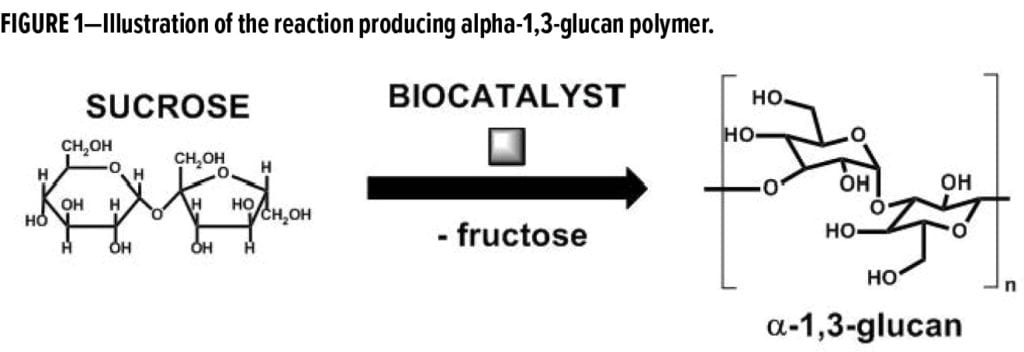
The alpha-1,3-glucan polymer generated in the enzymatic polymerization process is isolated as a water-insoluble, semicrystalline, highly structured material. Significant association through hydrogen bonding generates primary particles with a narrow particle size range (~10–30 nm), which further aggregate and agglomerate to form spherical particles in the range of approximately 500–5000 nanometers (Figure 2).
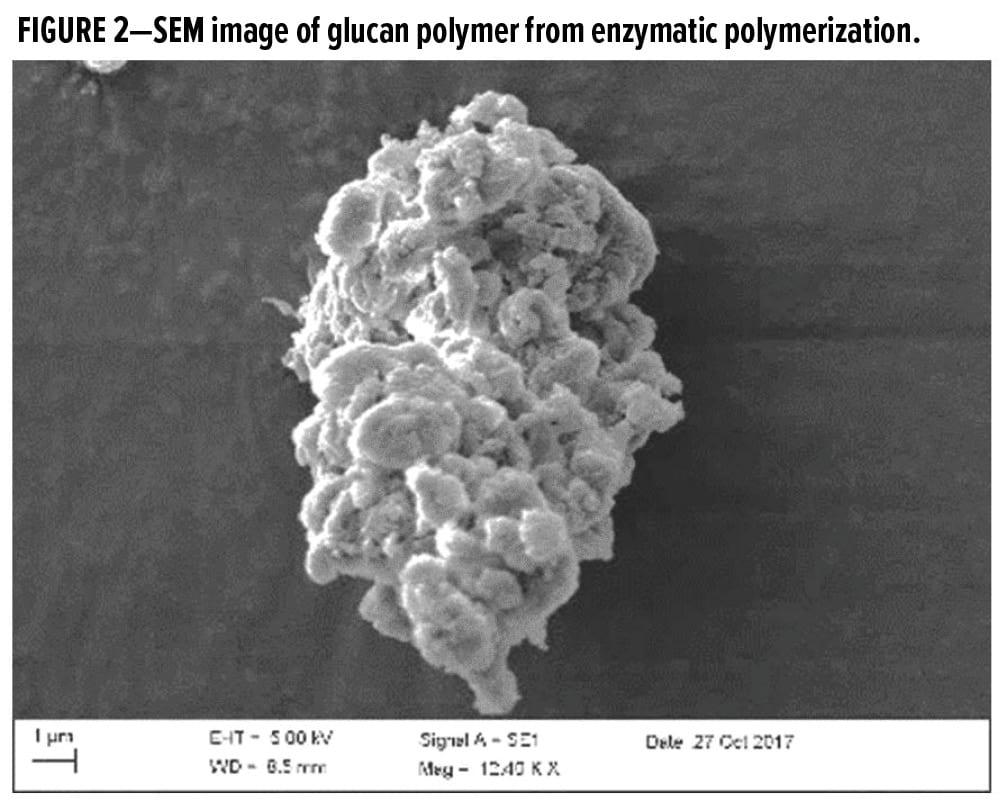
The polysaccharide isolated from this biopolymerization process is a free-flowing white powder that is water insoluble but water dispersible. It is hydrophilic with an open microstructure that is colloidally stable and can be redispersed under shear to form a colloidal dispersion in water, which shows high viscosity and is shear thinning. Polysaccharides in general are hydrophilic materials and will show a significant extent of associated water at equilibrium; however, the solubility in water will depend on the linkage, the molecular weight, and the degree of intramolecular chain association through hydrogen bonding. For example, linear dextrose (alpha-1,6-glucan), a typically water-soluble starch (blend of alpha 1,4 and 1,6 glucans), usually swells and expands in water, while typically cellulose (beta-1,4 glucan) though hydrophilic, remains undissolved in water and requires strong aprotic or coordinating solvent systems to generate true solutions. The alpha-1,3-glucan utilized in this work is also water-insoluble, similar to cellulose.
Figure 3 shows the shear viscosity of a 7 wt% colloidal dispersion of alpha-1,3-glucan in water. Black dots in the figure show the typical shear-thinning behavior of the material, while the shear stress of the sample is shown with red dots. At low shear rate, the shear stress shows a plateau, indicative of the yield stress required for the sample to flow.
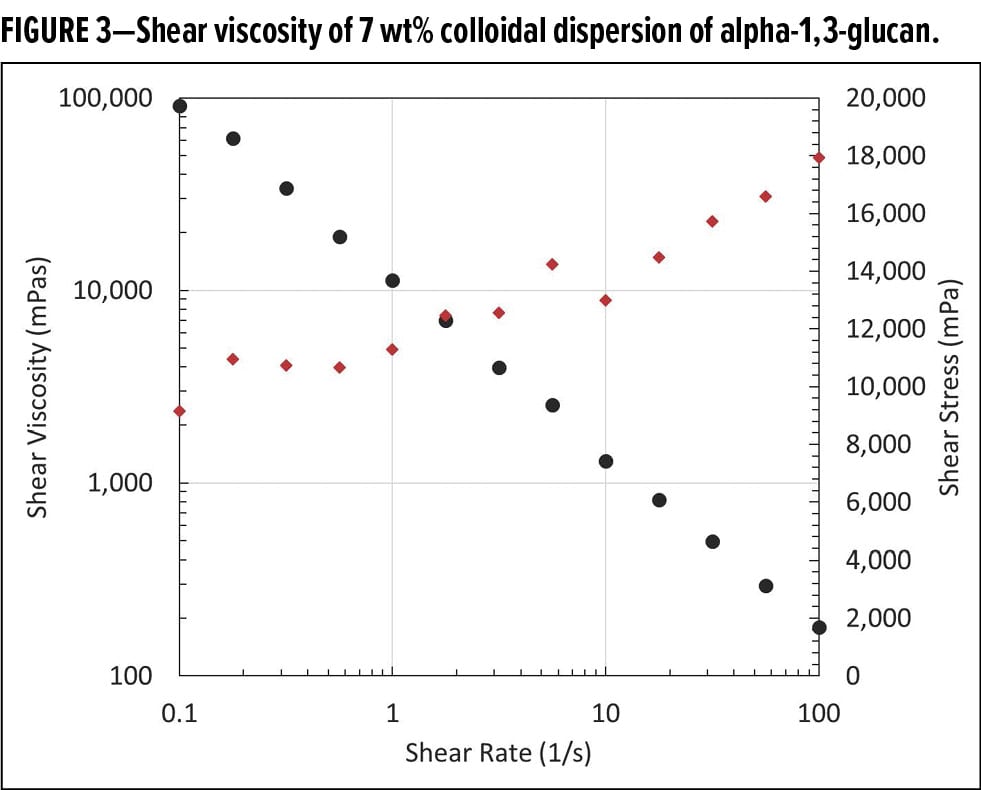
Figure 4 shows the viscosity of the colloidal dispersion increasing with solids loading. For concentrations > 10 wt% the system transitions from a flowing system to a soft solid.
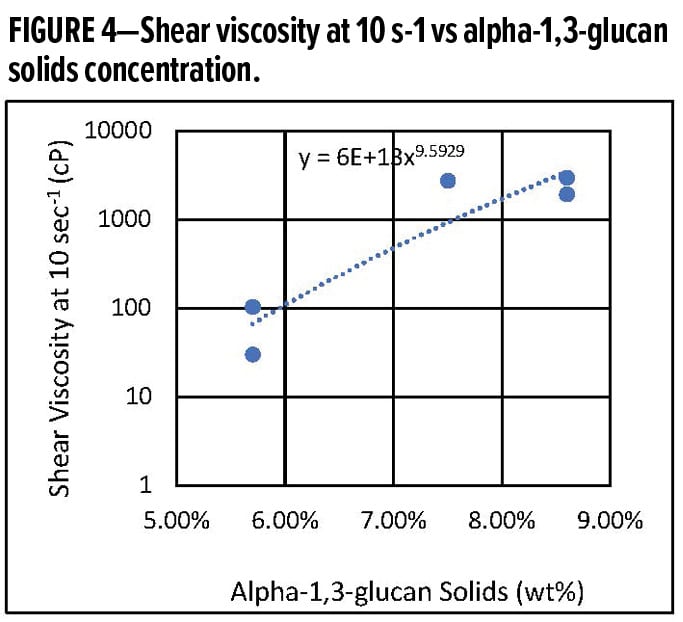
The transition from a flowable dispersion to a soft solid at low loading levels indicates that the spherical particles generate a highly structured phase.
The alpha-1,3-glucan is of high compositional consistency and purity and does not contain significant amounts of coproducts commonly found in other polysaccharide materials used for industrial applications (e.g., lignin or hemicellulose in cellulose products or various proteins in starch products) and typically contains < 0.2 wt% monosaccharide impurities. Based on the design of the enzyme, the process provides the target polymer with essentially exclusive selectivity, producing a linear polymer with greater than 99% alpha-1,3 linkages.2
Compatibility with Paint Systems
Based on the described characteristics of the alpha-1,3-glucan material to form stable colloidal dispersions in aqueous systems, the material can also be formulated directly into various waterborne resin systems used in the coatings industry. For example, acrylic latex, vinyl acetate ethylene (VAE) latex, alkyd resins, and epoxy resins all have been used in combination with this material to generate formulated systems.
High-shear mixers typically used in the paint industry with standard settings (i.e., Cowles blade mixer) are used to effectively disperse (or grind) the agglomerates of alpha-1,3-glucan into the latex or resin systems (i.e., VAE, acrylic, epoxy, alkyd), which generates well-dispersed and stable formulations with the polysaccharide with a typical particle size range on the nano to micron scale. Upon isolation, these structures will further aggregate to larger agglomerates. However, these latex or resin dispersions are homogeneous under application conditions and do not phase-separate (data not presented in this article), thus providing systems with excellent stability in typical formulations for paints and coatings.
The addition of dispersants or surfactants (i.e., siloxane surfactants, aminomethyl propanol) during grinding may aid in achieving the desired degree of dispersion and the associated desired particle size and stability. This ease of processing with standard industry application equipment and infrastructure is important for the utility and commercial viability of new materials in coating applications. The new polysaccharide material provides advantages compared to other emerging new materials (i.e., cellulose-based particle technologies) through the ease of processing into typical paint systems.
The objective of this work was to demonstrate the utility of alpha-1,3-glucan in architectural paint formulations, especially synergistic performance in combination with typical pigments.
EXPERIMENTAL
VAE Latex-based Interior Paint Formulations with Varying PVC—Model Architectural Paint Formulations
In typical latex formulations, improvements in rheology, opacity, tint strength, and whiteness are desired and are being demonstrated in new-generation formulations. Rheological properties of the paint formulations were measured using a Brookfield RVD viscometer. For optical properties (e.g., opacity, tint strength, whiteness), following ASTM D 2244-16, 3-mil wet drawdown films were prepared on standard drawdown cards (5.5 x 10 in.) and dried at ambient lab temperature for 24 h before analyzing the optical properties using an imaging spectrocolorimeter (X-Rite). After the optical analysis was completed, select drawdown films were used for cross-section scanning electron microscope (SEM) analysis (Figure 5).
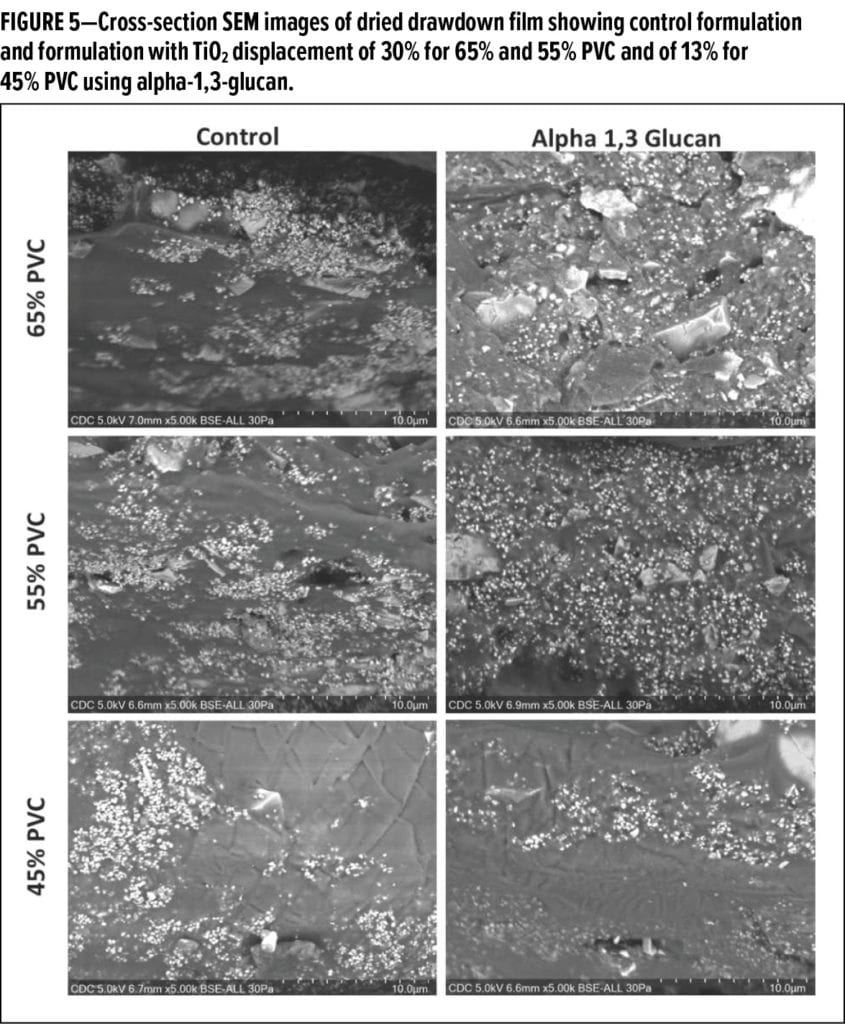
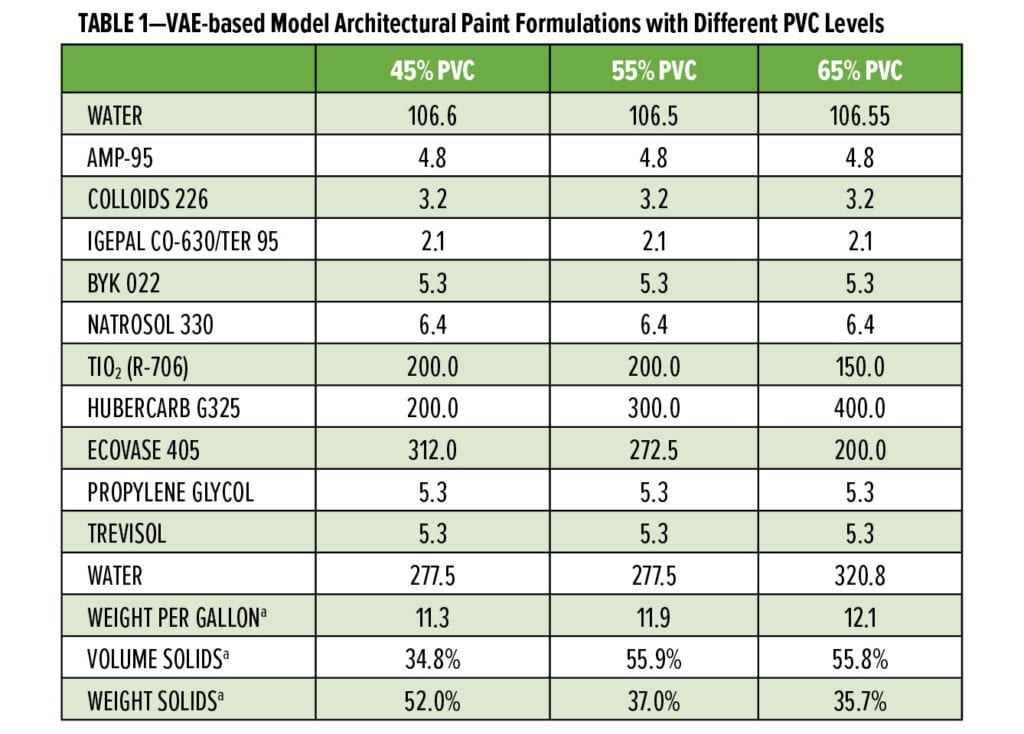
Table 1 shows the representative paint formulations for a white VAE architectural paint at three pigment volume concentration (PVC) levels.
Optical Enhancement and Displacement of TiO2
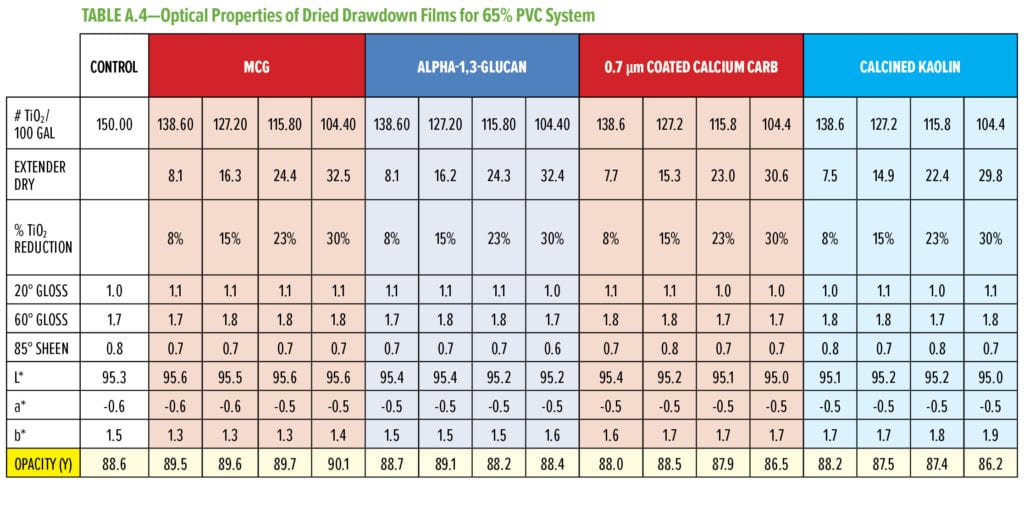
In this experimental design, the amount of TiO2 was displaced by the indicated amount of alpha-1,3-glucan designed to keep the overall PVC of the formula constant. Commercially available extenders (i.e., 0.7 or 3 mm calcium carbonate, hollow latex particles, calcined kaolin) were included in this study for comparative analysis. Optical properties, such as tri-angle gloss, L, a, b color values, and dry opacity, were measured on the cured films. Each point in the plots in Figure 6 (and Figures 7–9) signifies a single data point representing a specific formulation. The experiments were designed to evaluate the impact of alpha-1,3-glucan addition on the optical properties as the displacement of TiO2 was increased in the formulation. The data is considered indicative of the impact of the additives to the formulations, providing a consistent trend (as shown in the figures).
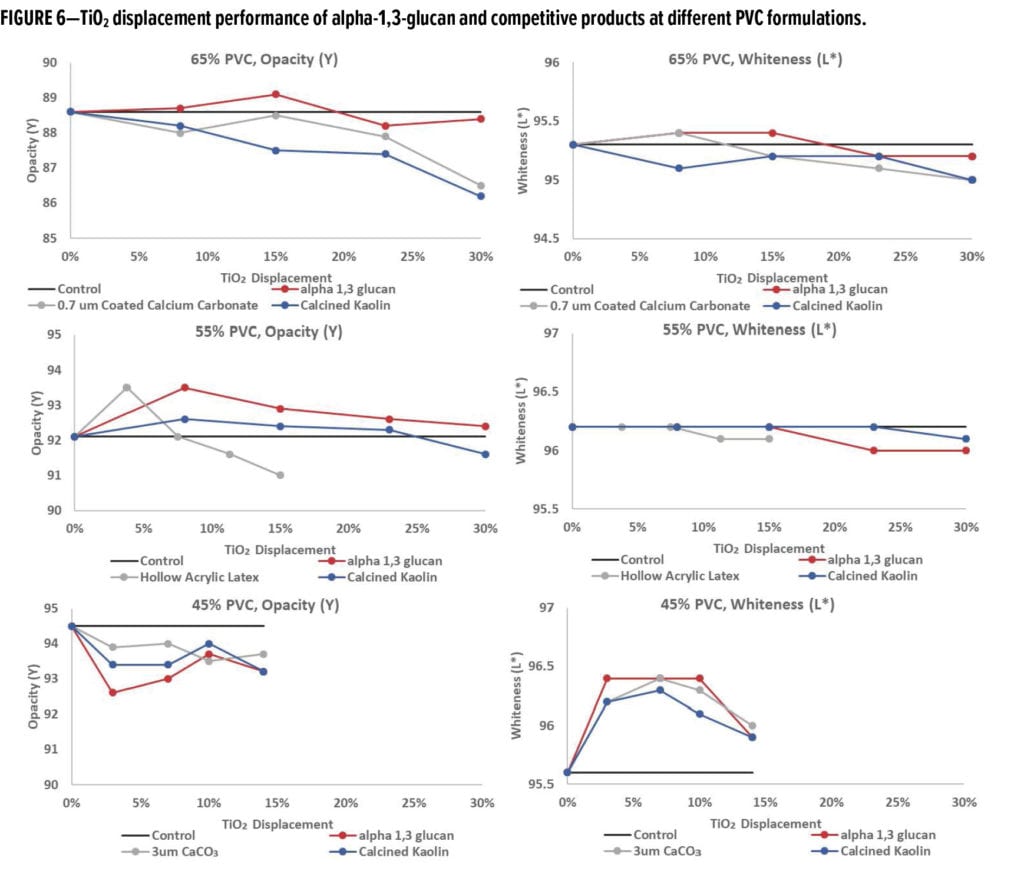
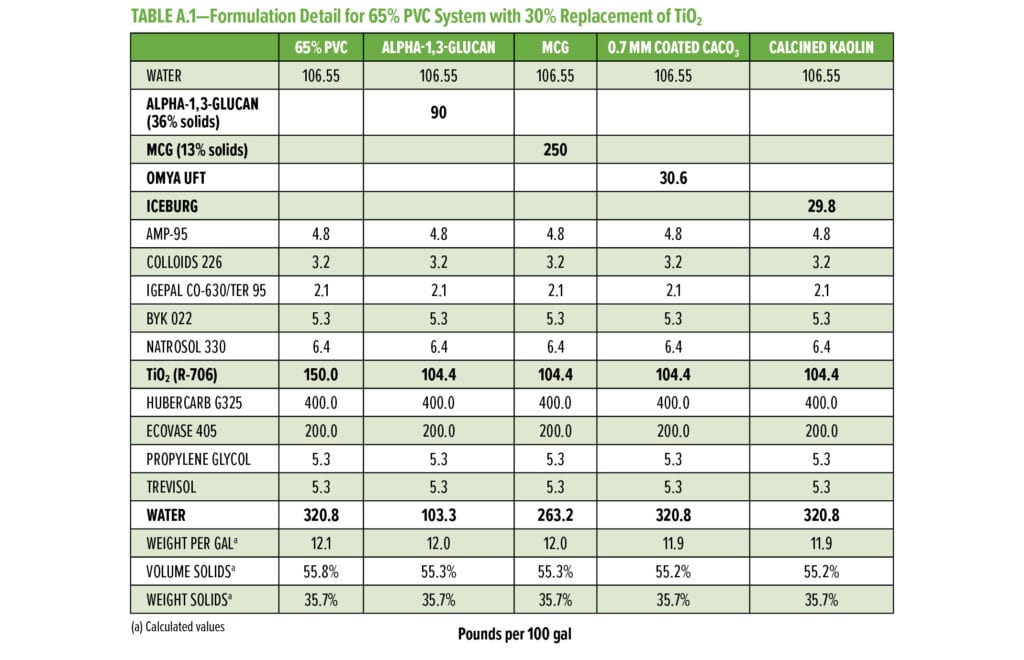
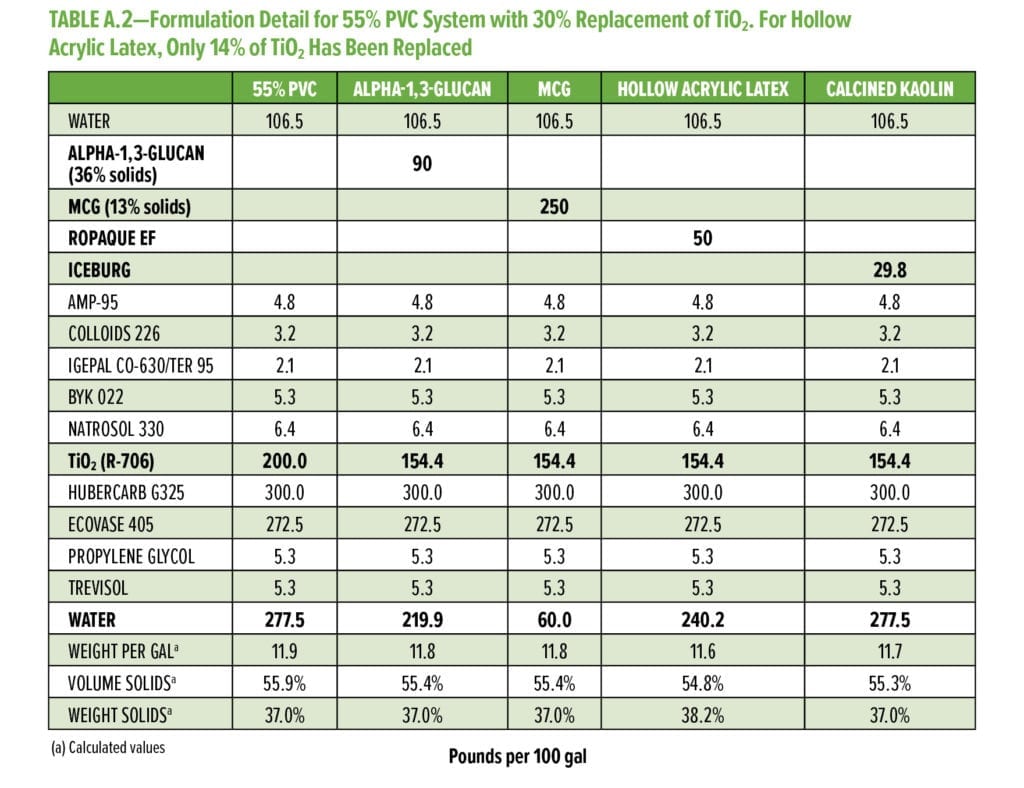
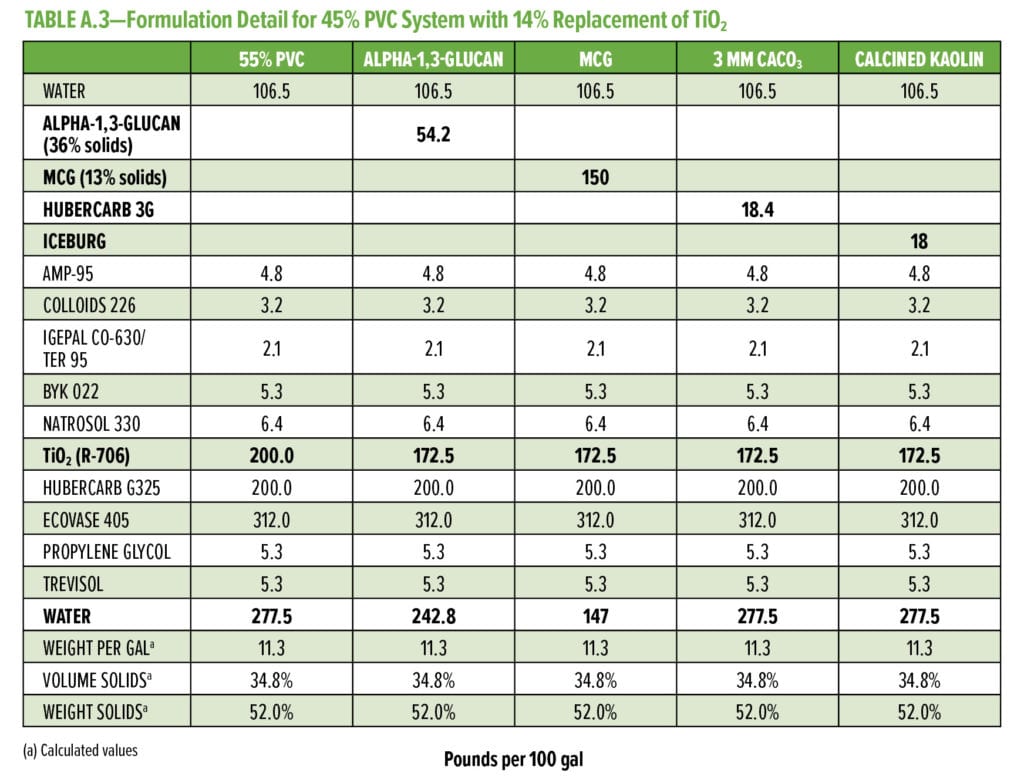
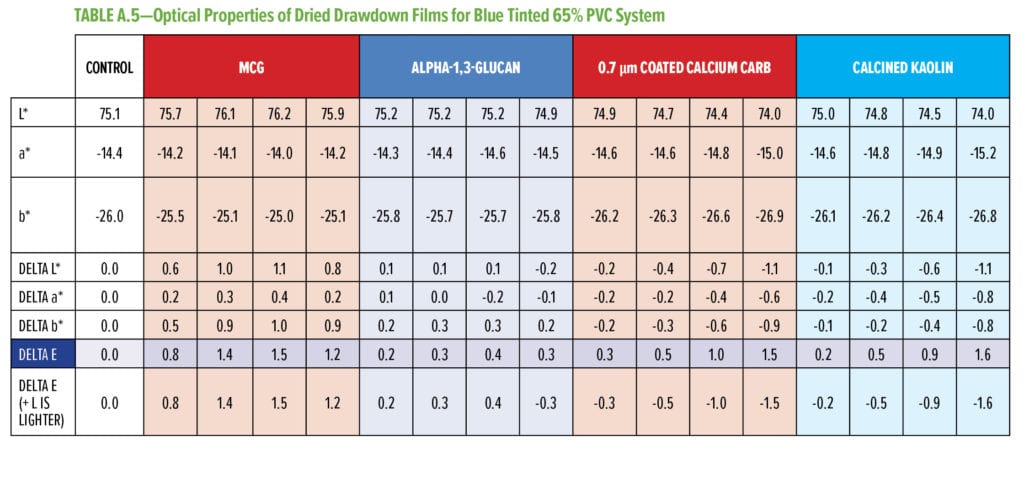
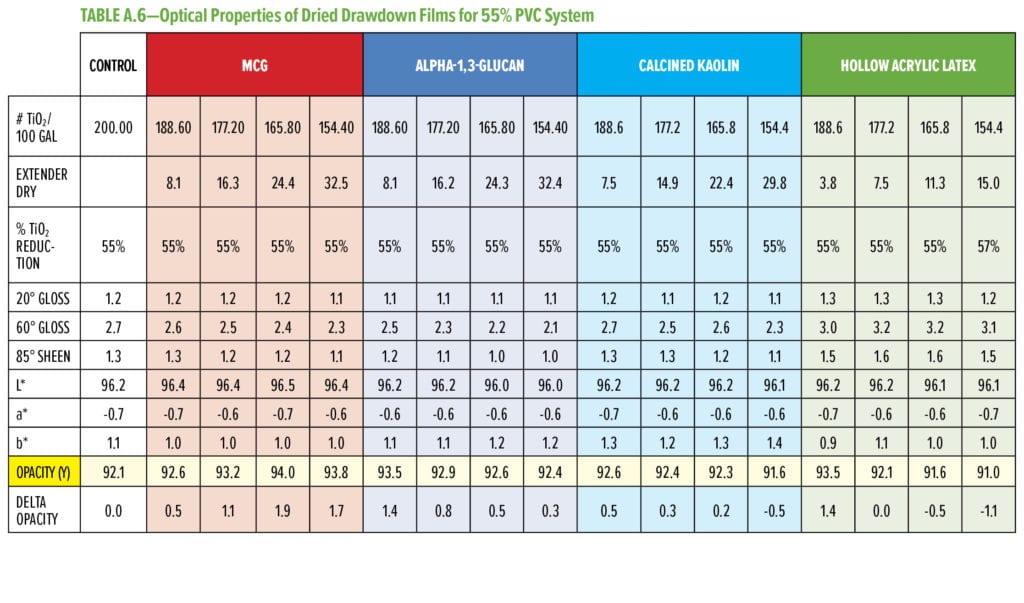
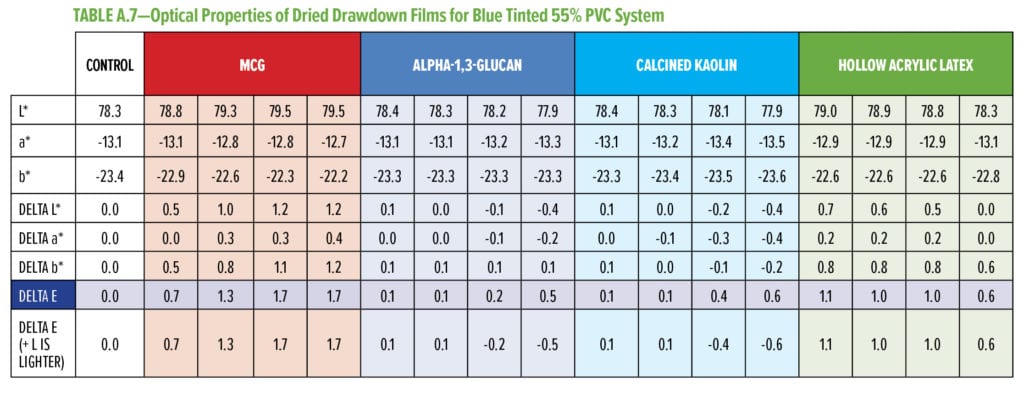
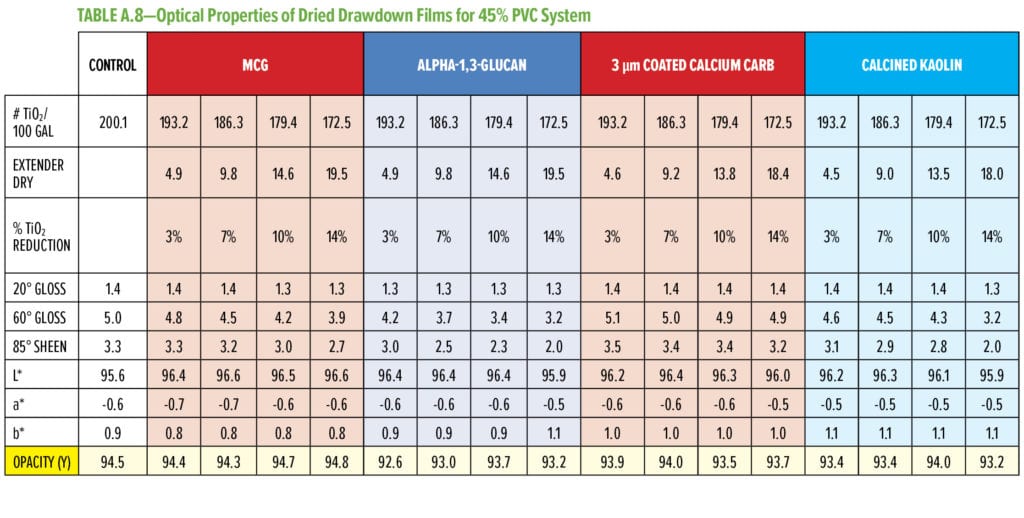
Typical additives used as TiO2 extenders will often also maintain optical properties at low replacement levels; however, as the extent of TiO2 displacement increases, the optical properties will quickly start to decrease. Interestingly, this is not observed as alpha-1,3-glucan is used in these formulations (Figure 6–9). As shown in Figure 6, for 55% and 65% PVC, the addition of alpha-1,3-glucan allowed for significant TiO2 reduction (30% reduction) without compromising whiteness (L*) or opacity (Y), whereas the additives used in comparison were able to only maintain the whiteness and/or opacity up to 8% to 15% TiO2 replacement. In fact, displacing TiO2 with an equivalent volume of alpha-1,3-glucan material-enhanced whiteness and opacity considerably, up to 23% TiO2 replacement for 55% PVC and up to 15% TiO2 replacement for 65% PVC. However, for the 45% PVC formulation both alpha- 1,3-glucan and alternative extender products were not able to displace TiO2 adequately while also maintaining opacity and whiteness. Full data tables on the analysis can be found in Tables A.1–A.9 in the Appendix.
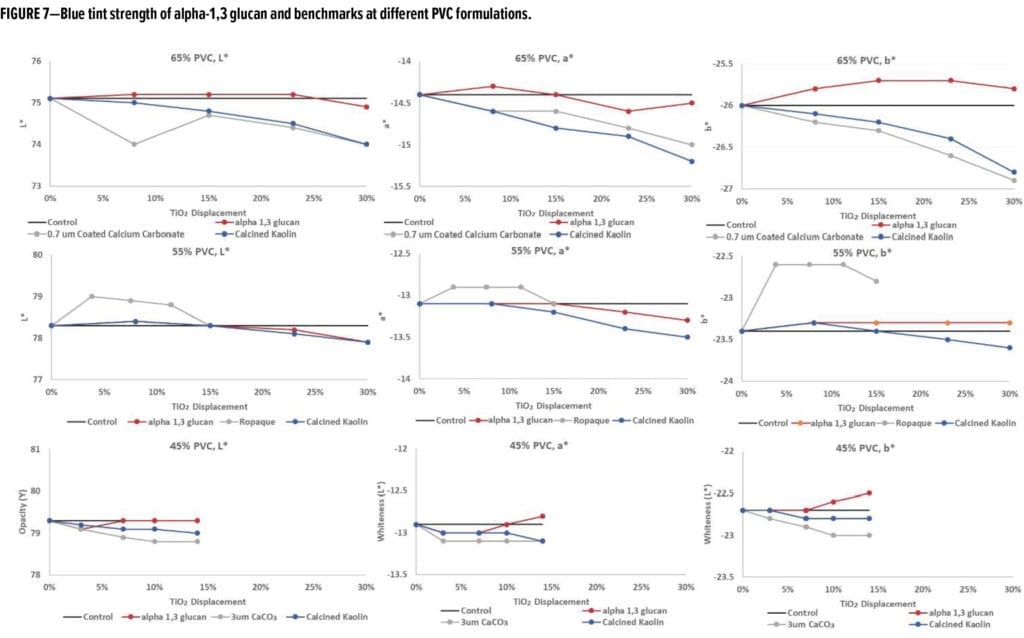
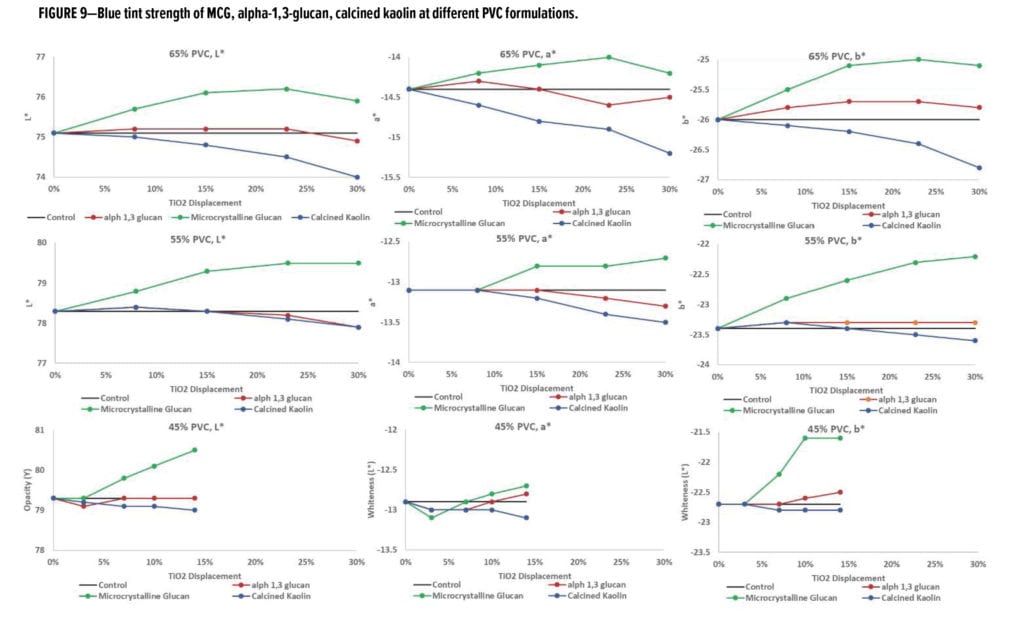
To assess the performance of these formulations with TiO2 displacement with regard to tinting, blue tint was added, and the optical properties of the dried drawdown films were analyzed, as shown in Figure 7. The blue tint strength data (Figure 7) shows that, for all three PVC systems, when alpha-1,3-glucan was used as a TiO2 displacement additive, the blue tint strength was very close to the control system and able to pass the typical quality control test used in the paint industry.*
To visualize the alpha-1,3-glucan’s mechanism of efficiently displacing TiO2, cross-section SEM images of the dried drawdown films were prepared as shown in Figure 5. The cross-sectional SEM imaging of cured films revealed that incorporation of alpha-1,3-glucan causes significant de-agglomeration and improved homogenous spacing of TiO2 particles throughout the matrix of the film for 65% and 55% PVC. For 45% PVC, although there is qualitatively improved spacing of TiO2 observed, the efficiency is much lower compared to the higher PVC% films. This supports the hypothesis that the glucan additive may function as an effective TiO2 extender, especially in high PVC paint systems.
The alpha-1,3-glucan formulation additive shows reduced efficiency of extending TiO2 at lower PVC. This may be based on the micron-scale particle size distribution of alpha-1,3-glucan as used in this format. Although the surface area of the alpha-1,3-glucan as measured was above 150 g/m2, the particle size averages around 5 to 10 mm, limiting the potential of extending TiO2 at low PVC. To further develop alpha-1,3-glucan as a more universal TiO2 extender with performance at all typical ranges of PVC, the average particle size of the alpha-1,3-glucan was further reduced to 0.5 mm through control of the bioprocess conditions. As the average particle size of alpha-1,3-glucan was decreased, the crystallinity index (XRD powder diffraction) was increased from 0.56 to 0.76. This increase in crystallinity suggests that the alpha-1,3-glucan chains show higher alignment, which will result in denser average particle structure at a fixed mass (decreased particle size). The engineered polysaccharide alpha-1,3-glucan can be formed in these two distinguished product forms, alpha-1,3-glucan and microcrystalline alpha-1,3-glucan (MCG) with lower particle size distribution and higher crystallinity.
The performance of MCG in displacing TiO2 under the same formulation conditions as described for alpha-1,3-glucan and benchmark products is shown in Figure 8. In comparison, alpha-1,3-glucan and calcined kaolin data are included and are the same data extracted from Figure 6. In all three PVC conditions, MCG consistently has a higher opacity and whiteness compared to controls including the lower PVC conditions (45% PVC). This suggests that further TiO2 reduction is viable without direct replacement through other pigments.
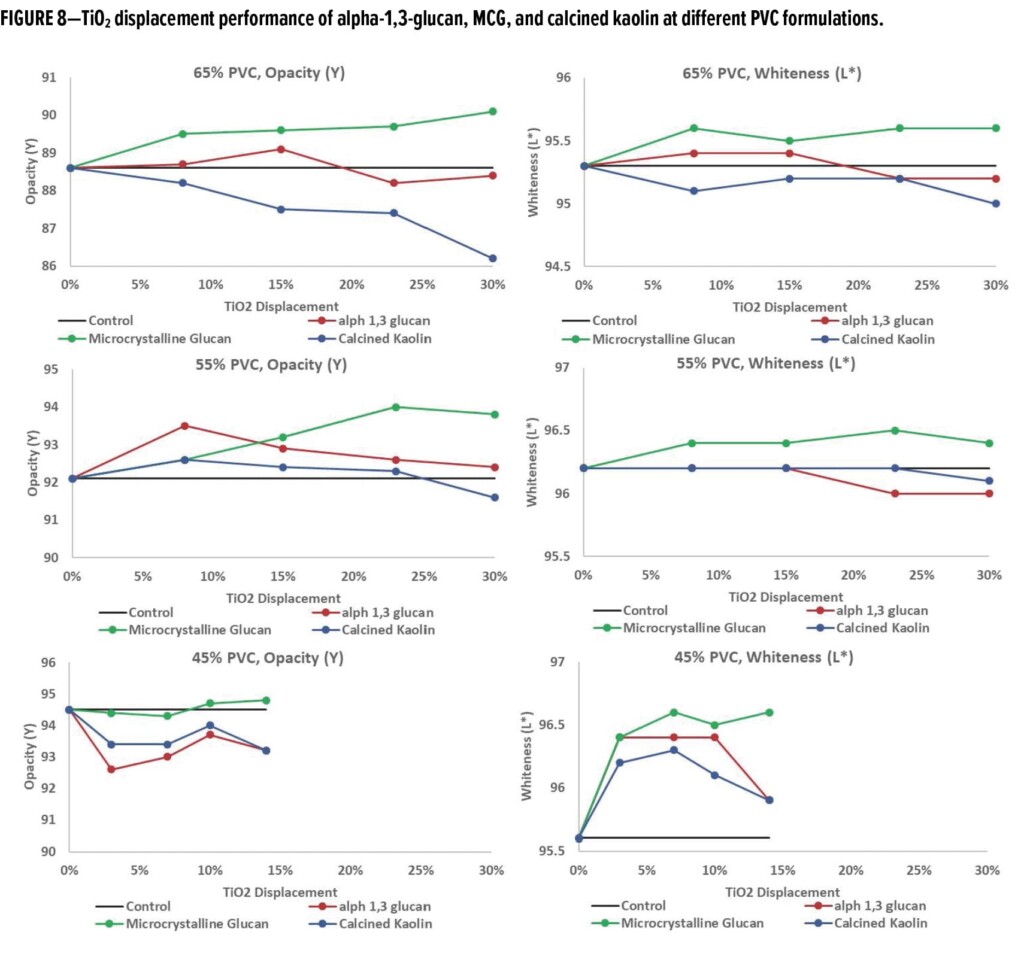
Similar to earlier evaluation results, the blue tint strength of MCG-containing formulations was compared to the control system as shown in Figure 9. In comparison, the alpha-1,3-glucan and calcined kaolin data from Figure 7 was included in Figure 9. Interestingly, for the examples using MCG, it was observed that for all three PVC systems the color of the blue-tinted system was “whiter,” suggesting that to obtain the target blue tint strength to match the blue tint strength of the control sample, the amount of TiO2 in the paint system required further reduction. This is consistent with the opacity and whiteness of the white paint formulation examples shown in Figure 8. In both situations, MCG had, in fact, overextended the TiO2.
Rheological Enhancement Properties of Glucan
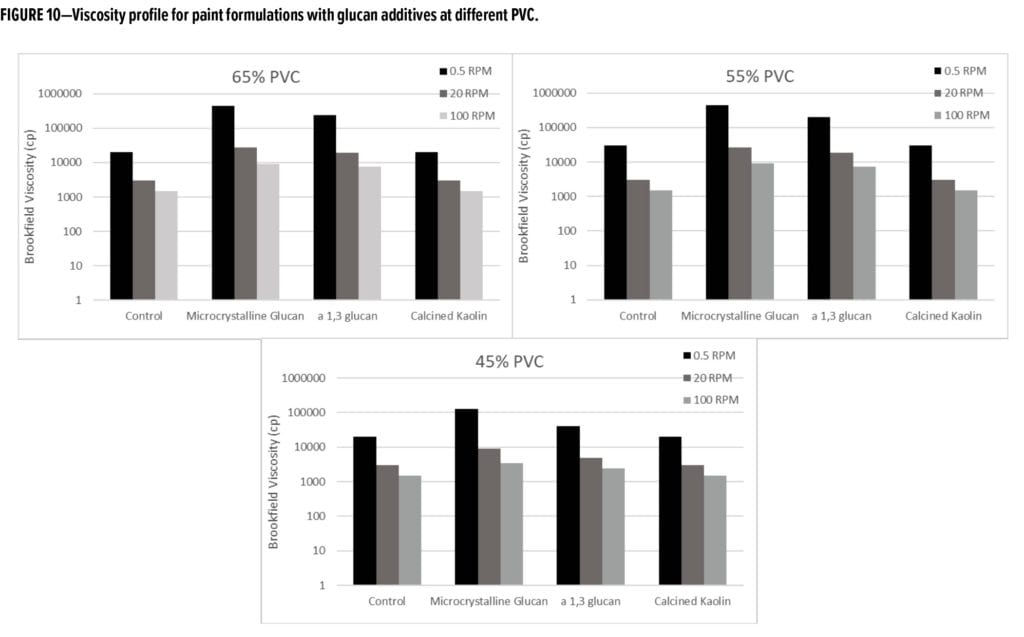
Both forms of glucan additives (alpha-1,3-glucan and MCG) also demonstrated a significant ability to increase viscosity at low concentrations in the formula in a dose-dependent manner, along with attractive shear-thinning rheological characteristics (Figure 10). This rheological activity of the glucan additives is anticipated to result in an overall reduction of other rheology modifiers typically included in the formula to control application performance.
Stain Resistance Properties of Alpha-1,3-Glucan Formulated Paints
Incorporation of the alpha-1,3-glucan materials into various architectural systems has consistently demonstrated that this additive does not negatively impact stain performance and, in certain instances, can even improve stain performance. Paint films utilized in the stain-resistance evaluation were prepared by drying the paint films on solid white drawdown cards for seven days at ambient lab temperature. Different stains were applied onto the dried paint film and were left to equilibrate for 10 min, then a dry paper towel was used to wipe away the excess stains. Next, a paper towel, wetted with nonabrasive ASTM scrub media, was applied onto a new dry paper towel, and the stains were rubbed for 10 double rubs or until a difference was noticed. Typical results are shown in Figure 11.
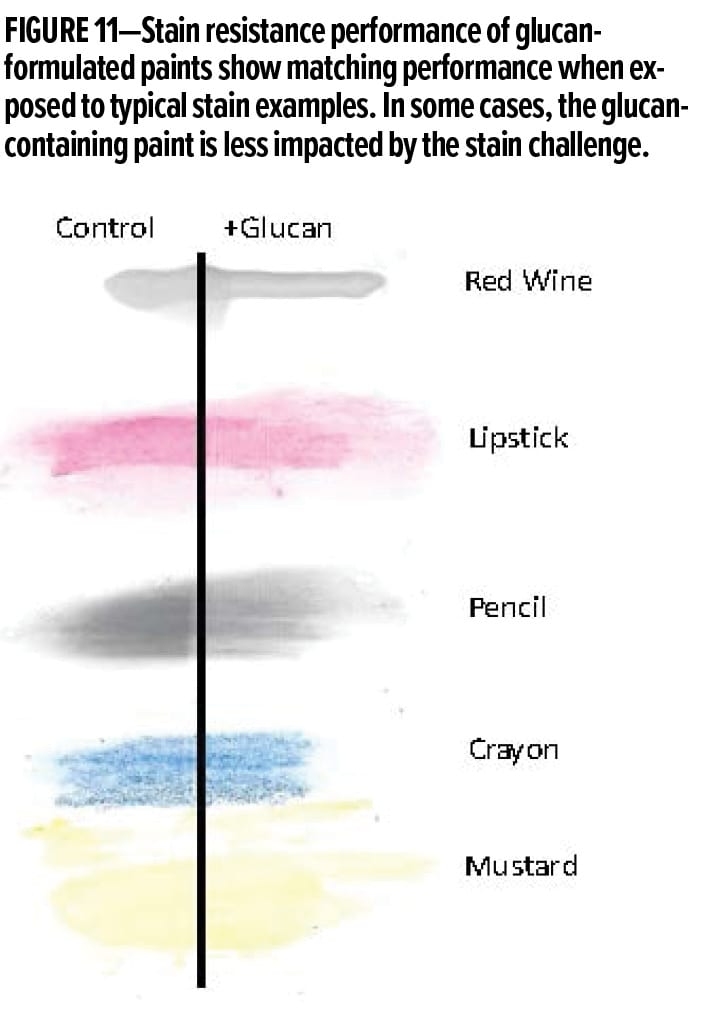
Performance of alpha-1,3-glucan-formulated paints show matching performance when exposed to typical stain examples. In some cases, the glucan-containing paint is less impacted by the stain challenge.
CONCLUSIONS
The engineered polysaccharide alpha-1,3-glucan accessible through enzymatic polymerization has been used in architectural paint formulations to demonstrate performance synergies. The alpha-1,3-glucan was provided in two particle morphologies, which were assessed in different PVC paint formulations replacing TiO2. Also, these additives were tested for their ability to modify the rheological and optical properties of waterborne architectural coatings. The findings presented in this article suggest that at relatively minor concentrations, the engineered polysaccharide has the capacity to function as a substantial rheological-modifying (thickening) agent with attractive sheer-thinning characteristics. In addition, it was observed that the engineered polysaccharide significantly enhances opacity, whitening, and tint-strength of a diverse range of latex systems. These optical enhancements may allow for the reduction of TiO2 without compromising the optical features of the paint.
The compatibility of the polysaccharide additive was demonstrated across a host of different latex resin systems and modifiers. The multifunctional nature of the glucan offers several degrees of freedom and considerable formulation-design flexibility, allowing formulators to adjust other components in the formula to balance physical performance and cost. A multifunctional material that offers rheology engineering, opacity building, whitening, and tint strength could be an attractive new additive for coatings where several important functional properties of the coating system can be directly improved using one additive technology.
References
- Habibi, Y., Lucia, L., and Rojas, O., “Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications,” Chem. Rev., 110 (6), 3479–3500 (2010).
- Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Derek, G., and Dorris, A., “Nanocelluloses: A New Family of Nature-Based Materials,” Angew. Chem. Int. Ed., 50 (24), 5438–5466 (2011).
Appendix
CoatingsTech | Vol. 17, No. 5 | May 2020
