By Weih Lee, Robert Polance, and Hart Haugen, The Sherwin-Williams Company
In this work centering around phenolic chemistry, we investigated isocyanate and benzoxazine-modified phenolic powder coatings for use in anti-graffiti applications and for addressing downhole drill pipe interior coating challenges. Allied polyurethane formulations were developed using phenolic hydroxyl-isocyanate reactions and shown to outperform conventional aliphatic polyol-isocyanate coatings for graffiti-resistant applications. Effects of variables like phenol hydroxyl equivalent weights and molecular weights of phenolic resins were examined. In a separate effort intended for oil and gas downhole pipe interior lining applications, novel fusion-bonded benzoxazine-epoxy-phenolic formulations, without or with an acid catalyst, exhibited excellent adhesion, toughness, and process-ability, in addition to greatly reducing cure requirements as compared to benzoxazine self-polymerization. Applicable rheology and kinetics of cure were quantified, revealing characteristic flow and cure behaviors to strengthen the understanding of structure-property relationships and to facilitate efficient optimization of coating performance.
Introduction
Phenolic resins are projected to reach $19.13 billion in global sales by 2026,1 growing as a core material in the formulation toolbox for a variety of industrial platforms, including molding compounds,2 foundry and sand casting,1,3 and coatings.1,4,5 Various phenolic grades consist primarily of novolac, resole, polyvinyl- and bisphenol A (BPA)-derived compounds, respectively. All of them readily crosslink epoxies due to the high reactivity of the phenol hydroxyl (Ph-OH). Resole phenolic resins, upon thermal activation, tend to homo-condense via a Diels-Alder ring-forming reaction of the methylol ether groups (–CH2–O–CH2–); hence, they are the preferred choice over the novolac resins for direct-to-metal primer formulations.6 Recently, lignin (amorphous bio-polyphenol)-based phenol-formaldehyde, and polyurethane resins have emerged in adhesives7 and even for agricultural fertilizers.8 In contrast to the phenol (C6H5OH) derivatives, BPA-based grades (Figure 1) are relatively new, stable, strictly para-structured with di-functionality of the acidic Ph-OH, and are suitable as curatives to formulate flexible and tough epoxy powder coatings. BPA-derived phenolic resins also have a less reactive secondary aliphatic hydroxyl (2nd-OH) within the repeating unit.
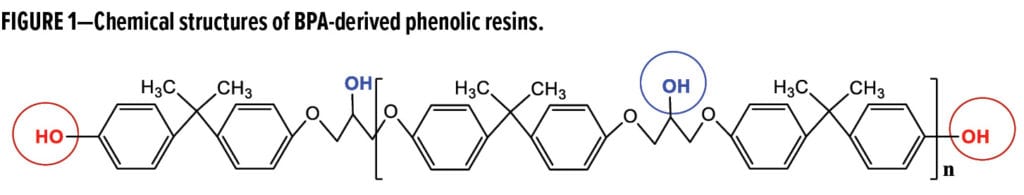
Polyurethanes (PU) are conventionally isocyanate-polyol reaction products bearing characteristic urethane (–NH–CO–O–) linkages. The polyol nomenclature predominantly refers to polyether (–O–), polyester (–O–CO–), polycarbonate (–O–CO–O–), acrylic, and tetrahydrofuran polymeric backbones containing multiple hydroxyl (OH) functional groups. Lately, OH functional polyamides (–NH–CO–) of stronger polarity have been used to improve adhesion. Most of these polyols have primary and secondary aliphatic OH groups, representing much weaker acids than Ph-OH and requiring elevated temperatures or catalysts to react with epoxies. Often, a PU topcoat, in conjunction with an epoxy basecoat, exhibits good weatherability and bonding strength. A zinc primer applied underneath is widely accepted for utmost corrosion protection,9 e.g., meeting ISO12944 C3-C5 specifications. The focus on phenolic PU in this work is within the scope of phenolic technologies in that reactive Ph-OH sources are exclusively from BPA-based phenolic resins (Figure 1) and aimed at fast-growing anti-graffiti markets dominated currently by silicone and PU chemistries,10 rather than from the traditional phenol resoles or novolacs that have been heavily used and confined to academic studies.11,12
Benzoxazines are regarded as next-generation phenolic resins13-16 that, when thermally initiated, undergo self-polymerization (i.e., ring-opening polyaddition, Figure 2), yielding a hard polybenzoxazine (PBZ) matrix. The rigid structure of PBZ comprises a) Mannich base bridges of CH2–N(R’)–CH2 instead of CH2 in conventional phenolic resins17,18 and b) a large amount of Ph-OH functionality facilitating intra-molecular H-bonding to strongly resist water.16,18
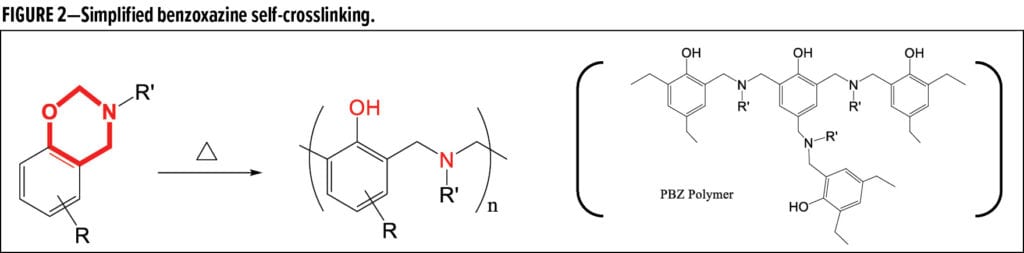
Meanwhile, the Ph-OH readily react with oxiranes to form benzoxazine-epoxy copolymers19 and with isocyanates to form benzoxazine functionalized PU.20 An enormous amount of research has been dedicated to benzoxazines and PBZ intended for various applications, such as electronic packaging and aerospace composites, including fiber-reinforced prepregs. However, there are few applied compositions of high-performance and low volatile organic compound (low-VOC) industrial coatings, particularly with respect to robust formulations or effective catalyzation to reduce benzoxazine cure temperatures or PBZ brittleness.13,15,21
Materials and Methods
Formulations discussed within the defined scopes are considered proprietary; therefore, their specific compositions may remain undisclosed. All resin materials and chemicals used in the current work were commercially available in a solid or free-flow form, including but not limited to:
- D5, D7, and D8 as three BPA-based phenolic resins (Figure 1) of varying molecular weights (MW) from 791, 1224 to 2282 g/mol by GPC-RI and phenol hydroxyl equivalent weights (EW-PhOH) from 250, 400 to 710 g/eq, respectively;
- An internally blocked isophorone diisocyanate (IPDI) uretdione (NCO dimers) having 310 g/eq EW-NCO (corresponding to 13.5% the total NCO content), 70~78 °C the melting point, 150~170 °C the de-blocking temperature, and non-smoking during oven cure;
- U0 as a branched polyester polyol resin of functionalities 5.60 and MW 2858 and the aliphatic hydroxyl equivalent weight (EW-OH) at 510 g/eq (corresponding to the hydroxyl number 110);
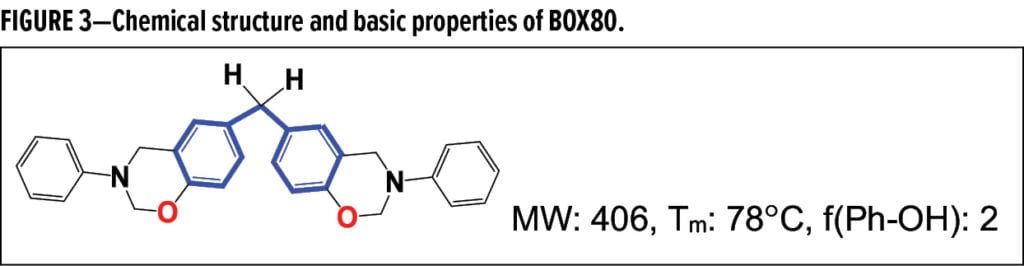
- BOX80 as a bisphenol F-based benzoxazine displayed in Figure 3; and
- Dibutyltin dilaurate (DBTDL) at 70% solids as a Lewis acid catalyst and p-toluenesulfonic acid (p-TSA) at ≥ 99% solids as an organic Bronsted acid.
Dynamic Mechanical Analysis and Differential Scanning Calorimetry
Viscoelastic properties during thermal cure were characterized by dynamic mechanical analysis (DMA) on a TA Instruments ARES-G2 rheometer using disposable parallel plates. Relative flow index during cure was obtained from the complex viscosity h* vs time curves at a fixed 7.0 °C/min heating rate along with the gel point, defined as the intersection of shear storage modulus G’ and loss modulus G’’. Thermal analysis was conducted on a TA Discovery DSC at 7.0 °C/min unless otherwise specified. Cure kinetic parameters were estimated from Differential Scanning Calorimetry (DSC) data by fitting them to the autocatalytic model equation
![]() (1)
(1)
Attained ordinary differential equations (ODEs) were solved using MATLAB® to allow the prediction of extents of cure (α’s) at selected times and temperatures. All Tg values were determined by either standard or modulated DSC.
Physical Testing
Adhesion was evaluated by de-lamination, blistering, softening, or swelling in any combination of the following two methods: hot water soak (HWA: 75 and 95 °C, 28 days) per NACE Standard Appendix J, and three-phase autoclave (177 °C, 3500 psi, and 24 h) as guided by NACE TM0174. Water uptake was assessed by weight gains in hot water soak. Toughness was compared by direct impact resistance per ASTM D2794 using a 4-lb weight with a 0.50-inch indenter at room temperature. Flexibility testing followed NACE Standard Appendix H: the mandrel four-point bend at -30 °C. Gel times at 204 °C and inclined plate flow (0.75 g pills) at
150 °C were carried out according to ASTM D3451/D4217 and D3541, respectively. Hardness and micro-scratch resistance under a constant load were measured by nanoindentation at ambient temperature. Taber abrasion with CS-10 abrasives and a 500 g weight applied were performed on coated Q-panels. Graffiti-resistant tests were implemented via a Method B protocol of clean-ability and re-clean-ability after markings left on panels for at least 24 h. Fourier-transform infrared spectroscopy (FTIR) was performed on cured films using nicolet iS10 at room temperature.
Results and Discussion
Proactive formulations herein concentrated on tackling functional barrier applications, such as graffiti-resistant coatings and drill pipe inner diameter linings. Anti-graffiti performance requires a combination of high hardness and gloss, resistance to scratch and to cleaner chemicals, and exterior durability. Downhole oil and gas drill pipe interior coatings demand high Tg values exceeding those at service temperatures, strong adhesion, moisture resistance, and toughness to sustain challenging high-temperature and high-pressure (HTHP) conditions. In the case of phenolic PU, BPA-based resins supplied aromatic OH groups to react with isocyanates, representing an alternative to existing aliphatic OH-functional polyesters for graffiti-resistant purposes. In the case of benzoxazines as an advanced phenolic resin, our investigation effectively demonstrated paths to more practical curing and processing conditions without compromising their distinctive structural performance.
Phenolic PU
Isocyanates (e.g., methylenediphenyl diisocyanate (MDI) or toluene diisocyanate (TDI) and polyols (e.g., polyesters) in a step-wise addition polymerization contribute hard aromatic urethane and soft aliphatic segments, respectively, imparting controlled hardness and elasticity in the resulting PU network. Similarly, di-functional BPA phenolic resins and internally blocked isophorone diisocyanate (IPDI) dimers were reacted to yield a thermosetting PU (Figure 4), where phenolic and urethane structures provide the hardened sections. Full characterization was conducted on a matrix of five formulations consisting of D5, D7, D8, U0-D8 (i.e., a 50–50 hybrid), and U0, a benchmark based on a commercial clearcoat polyester PU product for graffiti-proof applications without the addition of any functional stain- or mar-resistant additives.
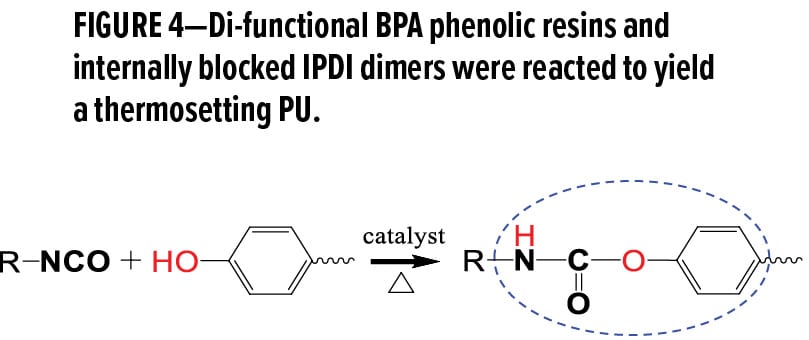
Other applied formulation parameters include: 1) a fixed stoichiometric ratio of NCO to OH at 1.025; 2) color tinted but unfilled; 3) outgassing agents and flow modifiers incorporated; and 4) identically catalyzed using DBTDL at 1.18 phr (parts per hundred resin).
Physical properties are reported in Table 1, and cure kinetics are reported in Table 2. D5, D7, and D8 denote individual formulations with phenolic resins of MW and EW-PhOH increasing in order. Property variations between an NCO to OH ratio of 1.050 and 1.025 were minimal.
FTIR-verified urethane linkages formed in each formulation, upon cure, by wavenumbers ~3347 (-NH-), ~1720 (C=O) and ~1235 cm-1 (N-C-O), respectively. Absorptions at 1507 (C=C) and 826 cm-1 (CH2=CH) attributed to aromatic rings within D8, D7, and D5 while missing in U0. U0 had fingerprints at 1100 (C-O-C) and 726 cm-1 (aliphatic C-C chains). All these signals appeared in U0-D8, as expected.
Results in Table 1 illustrate that phenolic PU batches outperform the polyester control (U0) in terms of 1) considerably higher glass transition temperatures, 2) increased hardness and scratch resistance, and 3) a glossier finish. Within the phenolic PU, Tg increased with MW or EW-PhOH, suggesting that the aliphatic 2nd-OH (see the blue circle in Figure 1) participate in the crosslinking reaction concurrently as the Tg is otherwise supposed to decline with MW. Increasing Tg represents increased crosslinking density developed during cure, improving resistance to chemicals and solvents such as graffiti cleaners. In formulating PU compounds, it is well known that one can increase Tg by incorporating a polyol with higher functionality (e.g., f = 10.4, MW 1972 and EW-OH 190). However, this approach impairs flexibility, also a desired feature. In addition, polyesters (–CO–O–) are structurally subject to detrimental hydrolysis that occurs slowly at ambient temperature but is accelerated in the presence of acids or bases. Phenolic polyurethanes are less susceptible to hydrolysis since they lack the ester linkage. Nanoindentation data (Table 1) demonstrate that hardness (H) and elastic modulus (E’) decrease with increasing MW or EW-PhOH of phenolic resins. Flexibility (deformation without fracture) and direct impact resistance (brittleness or toughness) of the phenolic PU were tested at 12 mil coating thickness on steel panels; results were comparable to U0, the polyester control.
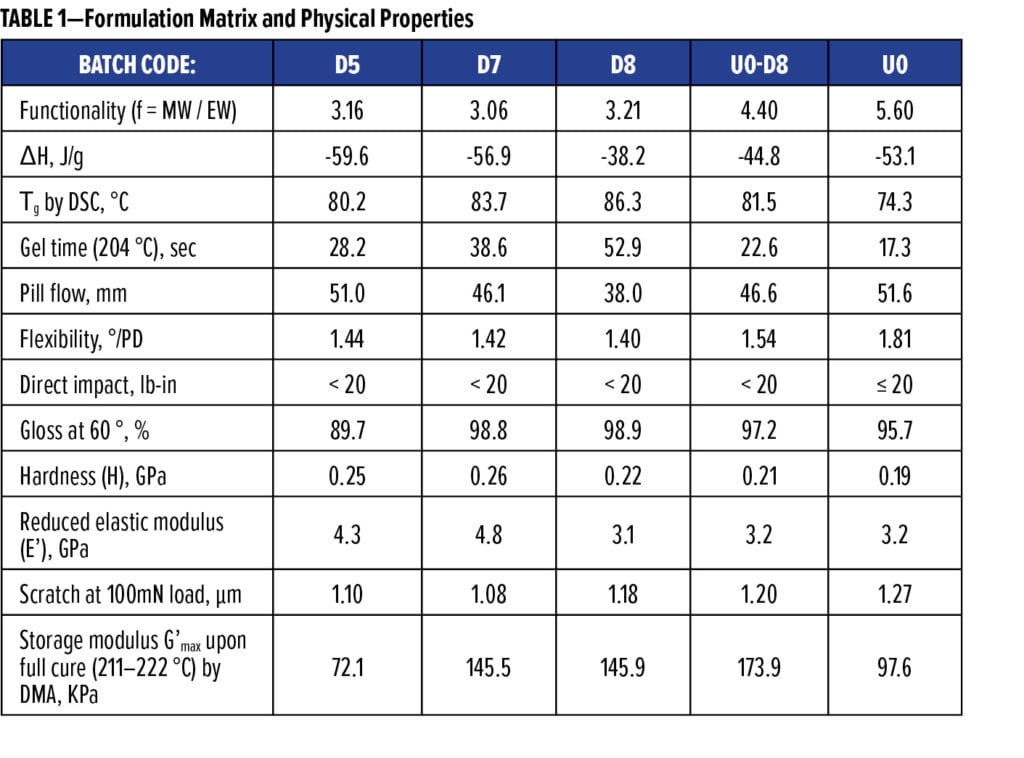
Functional coatings typically require hardness, elasticity (i.e., resilience), and toughness to provide scratch, abrasion, and impact shock resistance. The higher H and E’ of the phenolic PU, as compared to the U0, are ascribed to the initial MW and rigid benzene ring-contained structures of phenolic resins as well as the elevated Tg of the cured films. Micro-scratch data established that U0, at 1.27mm, is softest and comparatively the least scratch-resistant of the group with U0-D8 being between U0 and D8. Slight data transformation revealed that H, E’, and the actual scratch resistance are all inversely proportional to the functionality (f) of phenolic resins. Maximum storage modulus (G’max) from DMA, upon cure completion at 211–222 °C (Table 1), indicated that higher MW phenolic PU batches would withstand heat deformation better than the U0 control. The highest G’max up to 173.9 KPa was measured for the U0-D8 hybrid.
Taber weight loss is an indication of resistance to abrasion. Results in Figure 5 reveal that U0-D8 and U0 are equally abrasion-resistant, noticeably better than D8 and D7. Abrasion improved with increasing MW of phenolic resins or the addition of polyester resins in these batches. Weight losses, from small to large, were consistent with the ratios of H/E’, proposed to better describe abrasion resistance.22 Lower MW of phenolic resins (e.g., D5) or polyesters led to harder and more rigid cured films, resisting scratches better, but resulted in lower abrasion resistance. Meanwhile, higher MW resulted in softer coating films with improved abrasion resistance but not necessarily improved scratch resistance. For example, U0 was least resistant to scratches but withstood abrasion as well as or better than the U0-D8 hybrid among these formulations.
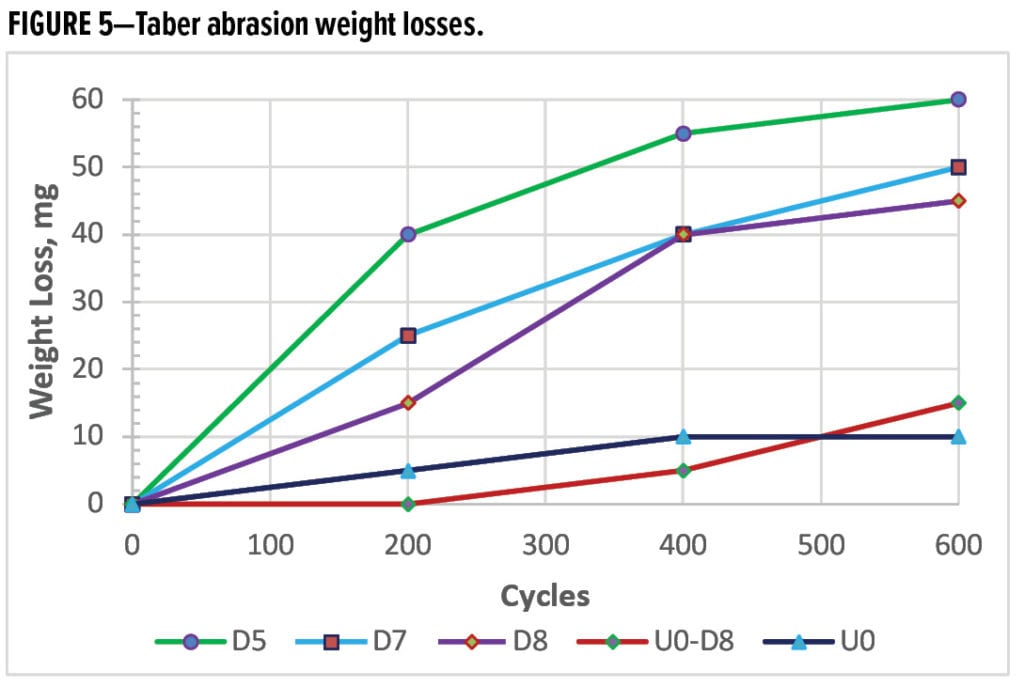
Cure kinetics were quantified (Table 2), including cure times and schedules predicted from the kinetic ODEs and verified by DSC on cured films. U0 gelled in 17.3 sec at 204 °C (see Table 1), the fastest among these batches, but it needed the longest time of 7’25’’ to complete cure because its curing reaction rates slowed down in the later stage post Tpeak (where the maximum cure rate occurs), as indicated by the reaction order of n, 0.69, the highest and the extent of cure (α) upon Tpeak at 69.5%, the lowest of the group. Cure times gradually increase with MW or EW-PhOH of phenolic resins; the greater the MW or EW-PhOH, the longer the cure time. D5, the lowest MW and EW-PhOH, cured fastest, as kinetic factors of the activation energy Ea, 56900J/mol, and the reaction order n, 0.65, were both lowest. Cure of PU free of competing reactions is kinetically much more straightforward than that of epoxies. The total reaction order for U0 was close to 1.25, nearly a first order reaction however delayed by uretdione endothermic de-blocking reactions prior to the NCO-OH reactions. Replacing the aliphatic polyester backbone with an aromatic phenolic apparently allowed the total reaction order (m + n) and Ea to shrink, kinetically promoting cure. As a result, phenolic-based PU improved cure completion through entailing either lower temperatures or shorter times to reach full cure.
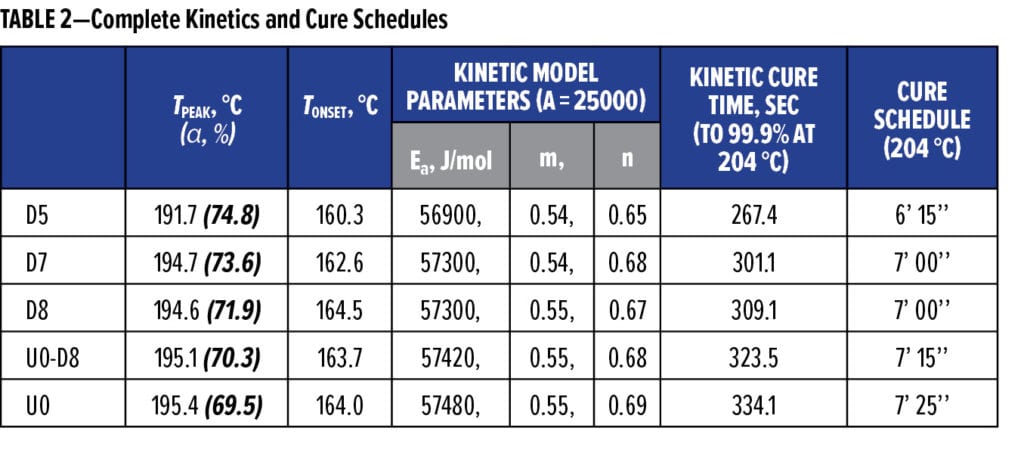
The minimum viscosity (h*min), gel and flow index (the area under the reciprocal h* vs time curve) were obtained for each formulation from rheology data. Under identical flow modification, U0 exhibited the lowest h*min (12.0 Pa-s) along with the highest flow index (21.3 1/Pa) and Tgel (the temperature at gelation, 178.8 °C) among these formulations. Increasing flow ahead of gelation during cure improves film forming and often adhesion. Considering phenolics, higher MWs with more molecular entanglement led to less flow and lower Tgel. In comparing the extent of crosslinking at h*min and the gel point, U0 gelled at α = 25.6% with h*min at α = 7.7% while the phenolic batches and U0-D8 all gelled far before h*min taking place. U0-D8 displayed profiles (Figure 6) representative of rheological behaviors of the phenolic-based formulations; it gels to form an immobilized network at 154.3 °C first, reaches h*min at 164.4 °C next, and undergoes substantial crosslinking until cure completion at about 220.9 °C, where G’max is at the highest value. Pill flows (Table 1) were very consistent with rheological flow indexes.
The film integrity and full gloss finish of the higher MW cured phenolic PU films were found to be as good as or better than the polyester control, U0. U0-D8 offered synergic interactions from the combination of aromatic and aliphatic OH groups exhibiting better flow, gloss, enhanced hardness, modulus, and abrasion, mitigating potential hydrolytic degradation, and most importantly in promoting cure and increasing Tg for better chemical resistance. These upgraded qualities serve to improve graffiti resistance. Assessed per anti-graffiti specifications, D7, D8, and U0-D8 were rated 1 (completely removed with dry cotton cloth) in clearing blue wax crayons, lipsticks, and black water-based ink markers, very comparable to U0. When dealing with blue permanent markings, the referenced phenolic PU were all rated 4 (completely removal with IPA) vs U0 that was rated 5 (completely removal with more aggressive acetone); each of them retained or resumed full gloss after cleaning. Please note, all formulations were preliminary, free of any stain-resistant additives, although the U0 binder system was based on a commercial anti-graffiti product. Performance should further improve with formulation optimization of the stoichiometric ratio for the Ph-OH and the 2nd-OH. Besides, phenolic resins themselves are natural antioxidants, which should aid in mitigating the need for other stabilizers. Hybrid systems may afford better UV-light stability than 100% phenolic PU for weatherability purposes.
Fusion Bonded Benzoxazines
Fusion bonded epoxy (FBE) powder coatings, formulated with amine or phenolic curatives, are one of leading solutions to functional and protective applications such as pipeline, rebar and valves. A possible forward evolution of FBE technologies, benzoxazines could improve traditional FBE formulations, enabling their use in more demanding applications. An initial evaluation of these self-cross-linkable materials (see Figure 2) was conducted on BOX80 to better understand their properties. BOX80 self-polymerization generated a record high exotherm of –306.6 J/g resulting in Tg of up to 186.8 °C (Figure 7). The cure path was found to closely follow the applied autocatalytic model, like that of most epoxy thermosets. Along with the reaction orders of 0.96 for m and 1.42 for n, BOX80 homo-polymerization, heated at 7.0 °C/min, yielded 55.55 KJ/mol activation energy (Ea), a high value close to some epoxy formulations or products per statistical data. A narrow cure window from 210 to 270 °C, estimated by the baseline crossovers, revealed that BOX80 remained highly latent and stable until the cure temperature (Tcure) surpassed ~215 °C, where polymerization rapidly proceeded until gelling at 233.9 °C (Table 3); the cure rate peaked around 234.6 °C immediately after the gel point, and cure completed at ~273.0 °C.
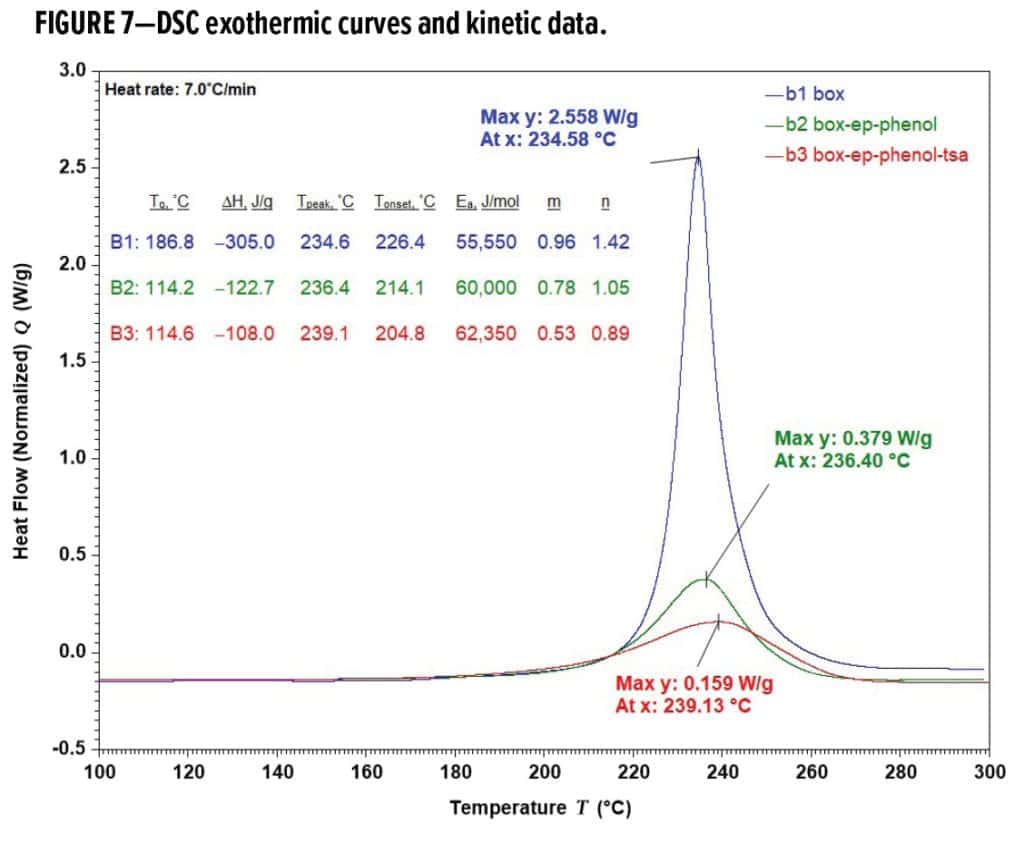
The majority of BOX80 self-curing (i.e., 84.4% of the total exotherm) took place between h*min at 226.3 °C and h*max at 244.3 °C within a short period of 2.6 minutes around gelation at 233.9 °C (Figure 8). BOX80 softened and melted at ~80 °C, creating a massive flow and low dynamic viscosity (h*) consistently below ~10Pa-s until 226.3 °C. The wide flow window between ~80 °C and 233.9 °C (the gel point) provides a strong potential for substrate-wetting adhesion. Starting h*min at 226.3 °C, h* experienced a sudden jump throughout h*max, signifying prompt cure upon thermal initiation. BOX80 was already 8.60% cured at 226.3 °C kinetically; therefore, it is misleading to assume the exact turning point of h*min in Figure 8 as the actual cure or gel onset. Instead, it indicates just the dynamic inception of rapid h* buildup. Viscous and elastic components (G’’ and G’) of BOX80 co-dominated until the gel point, where elasticity started to dominate as G’ > G’’. BOX80 gelation happened at α = 50.2%, after h*min and right before Tpeak at 234.6 °C and α = 55.2%. h*max occurred at 244.3 °C and α = 93.0%, corresponding to in-situ G’max as high as ~2.08 MPa.
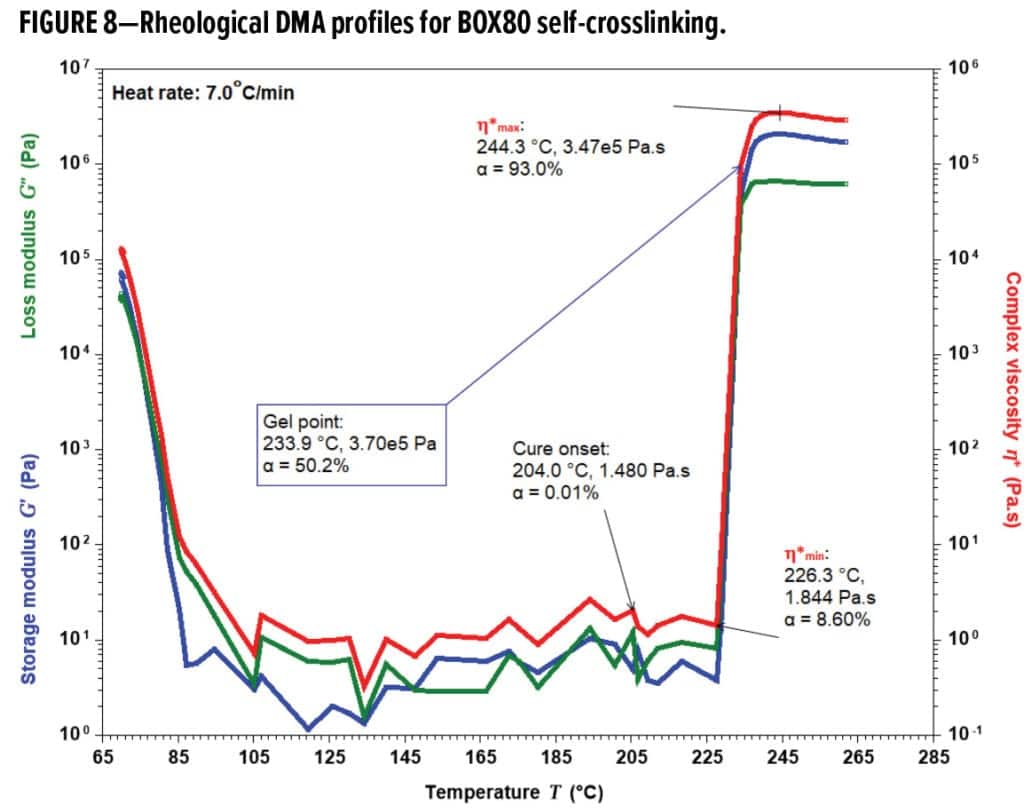
The unique kinetic and rheological traits observed for BOX80 homo-crosslinking prescribed that consequent formulations must overcome inherent difficulties firsthand to perform robustly. One challenge was a water-like melting viscosity (h*) giving a flow index of 206.4 1/Pa and a pill flow of over 200 mm (B1 in Table 3), causing severe sag when applied. Incorporation of flow control agents along with fillers assisted in managing this flow issue effectively. Flow index and pill flow were reduced to ~46.0 1/Pa and ~90mm (Formulations B2 and B3 in Table 3) at 25.6% filler loading, respectively, resulting in fully processible and sag-free composition without loss of adhesion.
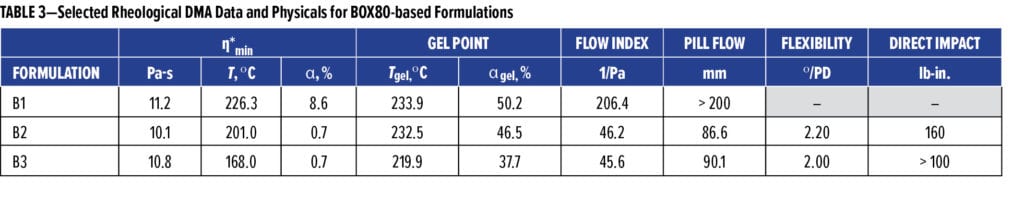
Another concern was the severely high Tcure required for BOX80, exceeding 240 °C, with 260 °C recommended for full cure. Reducing Tcure and cure times would significantly increase the commercial applicability of benzoxazine systems. Encouragingly, implementation of blending BOX80 with epoxy and phenolic resins at a stoichiometric ratio of 100:60:20 efficiently reduced the cure time from 19.4 to 13.1 min at 232 °C (Formulations B1 and B2 in Table 3) as determined from kinetic modelling and confirmed by cured film DSC. However, cure time reductions came at the expense of a sharp Tg loss from 186.8 to 114.2 °C, implying substantially diluted crosslinking density in B2 (BOX-Ep-Phenol), where a copolymer of benzoxazine-epoxy was supposedly formed instead of PBZ alone. Although physically maintaining the high Tg, combining BOX80 with only an epoxy resin was ineffective to reduce cure settings. BPA-based or novolac epoxies performed equally. The epoxy/phenolic modified BOX80 formulation led to even higher latency with Ea at 60.0 KJ/mol (vs 55.6 KJ/mol for BOX80). Remarkably faster cure responses of B2 were overall a kinetic consequence of the reaction orders dropping from 0.96 to 0.78 for m and from 1.42 to 1.05 for n, respectively.
FTIR spectra were consistent with benzoxazine self-condensation for B1 and benzoxazine-epoxy copolymerization for B2. A strong and broad absorption at ~3630 cm-1 correlates to OH and H-bonding from the PBZ matrix in B1. The B-staged powder of B2 was almost identical to that of general epoxy/phenolic formulations, displaying an intense and sharp fingerprint signal at 1139 cm-1, characteristic of ethers (C-O-C). However, this absorption became too faint to see in cured films of both epoxy/phenolic systems and B2. Meanwhile, the OH-related signal from the cured films of B2 were too weak or even disappeared, inferring that phenol OHs (from phenolic and PBZ) were almost depleted from reacting with epoxies.
In physical testing, B2 showed outstanding impact resistance, greater than 100 up to 160 lb-in. at 12–14 mil on blasted steel panels. Water uptake upon hot water soak for 28 days at 75 and 95 °C averaged 0.31 and 0.90 g (compared with generic FBEs of a similar Tg ranging typically from 0.5~1.0 and 1.5~2.5 g). The direct-to-steel single coat application of B2 passed autoclave testing at 177 °C free of any blisters or delamination or softening or swelling. In contrast, current FBE systems with Tg higher than test temperatures require a liquid primer to achieve similar HTHP performance. The fusion-bonded BOX80 modifications have the following implications: First, BOX80, if properly combined with an epoxy and a phenolic, renders superior structural adhesion in the absence of primers. Second, high Tg are not necessarily a prerequisite for some difficult HTHP applications such as downhole drill pipes.
Use of catalysts is another approach to ease BOX80-required cure conditions. Common tertiary amine catalysts such as 2-methyl imidazole, 4-dimethylaminopyridine, amine adducts and imidazole adducts, all effective accelerators for epoxy oxirane ring opening, were surprisingly ineffective in BOX80 formulations regardless of their loading levels or combinations. Other benzoxazine grades responded similarly, suggesting that basic or anionic materials not be suitable catalysts for these benzoxazine ring-opening reactions. Subsequently, a strong organic acid capable of proton donation was evaluated. Unblocked p-TSA was chosen as an acid with better thermal stability and compatibility than inorganic acids and was found to perform well, as represented by B3 (BOX-Ep-Phenol-TSA), where the cure time at 232 °C was reduced to 11.8 min. Kinetically, p-TSA worked by further driving the reaction orders down to 0.53 for m and 0.89 for n, respectively, while increasing Ea to 62.35 KJ/mol. Overlapped DSC exothermic curves are shown in Figure 7. The cure window for B3 as a result of p-TSA addition shifted further to a broader temperature range of 160.7~276.2 °C vs 161.0~283.0 °C for B2 and 177.7~273.0 °C for B1. Impacts of catalytic p-TSA incorporation on mechanical toughness, flexibility or Tg were minimal (Table 3).
p-TSA was effective in the presence of a phenolic resin, regardless of whether an epoxy resin was in the formulation. The optimal loading of p-TSA was determined to be at approximately 1.21 phr, with the DSC Tonset at 204.8 °C, at least 10 °C lower than 0.40, 0.81, or 1.61 phr. This is likely related to its decomposition temperature of ~170 °C, significantly below Tcure (e.g., 232 °C). p-TSA was most effective in benzoxazine-phenolic systems free of epoxy resins, reducing DSC Tonset from 204.8 to 158.7 °C at 1.21 phr and retaining high Tg but sacrificing impact resistance to a level comparable to that of conventional epoxy systems (i.e., ≤ 40). Other organic Bronsted acids, such as dodecyl benzenesulfonic acid or Lewis acids (electrophilic or e- acceptors), either blocked or un-blocked, with higher reactive temperatures should also serve well as catalysts for benzoxazines.23,24 Preliminary work with DBTDL as a Lewis acid catalyst to expedite BOX80 curing was also positive, suggesting that applied isocyanate-cured benzoxazine systems be feasible using a co-catalyst. An initial batch of B3 where an isocyanate substituted the epoxy also produced interesting results. Nevertheless, it was unsuccessful to apply p-TSA as a catalyst in the PU systems, as tested.
Conclusion
BPA-based phenolic resins and benzoxazine monomers were introduced into polyurethane and fusion-bonded epoxy powder coatings resulting in enhanced properties to cope with anti-graffiti and downhole drill pipe interior challenges, respectively. The structure-property relationships in functional coating formulations were examined, indicating that compositions of initial resins, polymerizable ingredients, and cured networks are equally crucial to end-use attributes such as Tg, hardness, modulus, adhesion, toughness, etc. Robust coating performance requires effective processing and optimized cure, qualifiable and quantifiable through a combination of rheology and kinetics.
BPA-based phenolics, when partially or completely replacing aliphatic polyols in polyurethane powder coatings, improved Tg and chemical resistance, film hardness, scratch resistance, and cure completion, providing property enhancements suitable for graffiti-resistant functions. Structural characteristics, aromatic rings, molecular weights and phenol hydroxyl equivalent weights of BPA-based phenolics dictate end coating performance with higher MW or EW-PhOH positively affecting Tg, cured film integrity, gloss, and abrasion resistance. Phenolic polyurethanes were observed to gel before minimum complex viscosity (h*min) in contrast to gelation behind h*min for conventional polyol PU. Formulation optimization should include a rebalance of the stoichiometric ratio to cover the aliphatic 2nd-OH. Hybrids of high MW phenolics and polyester polyols allowed advantageous interactions in solvent and moisture resistance.
Appropriately formulated benzoxazine powder coatings attained sag-free processability and milder cure settings to deliver direct-to-steel single coats with outstanding adhesion, cohesive toughness, and moisture resistance, highly resisting direct impact shocks and passing HTHP autoclave testing, although Tg dropped under 120 °C. Incorporation of epoxy resins yielded a tough heterogeneous benzoxazine-
epoxy matrix, maximizing impact resistance while maintaining excellent moisture resistant performance. The addition of phenolic resins carrying acidic Ph-OH was qualified to lower the kinetic reaction orders while enhancing latency, allowing cure at lower temperatures and/or shorter dwell times. Organic Bronsted acids as proton donors were suitable catalysts in cationically promoting the benzoxazine ring-opening polymerization; in contrast, epoxy C-O-C ether ring-opening reactions are typically anionically catalyzed by Lewis bases such as tertiary amines. p-TSA catalyzed benzoxazine-phenolic systems reduced cure times additionally. Other Bronsted or Lewis acids, depending on heat stability, are similarly effective benzoxazine accelerators. Structure-driven adhesion, cohesion, modulus, and film impermeability are more critical bulk properties than high Tg on its own to meet or exceed strict HTHP applications. Benzoxazine systems offer significant promise as a platform comprising two-layer powder systems to match or exceed the long-lasting performance of three-layer liquid systems adhering to ISO12944-C5M specifications.
Acknowledgments
The authors extend thanks to Rafael Reyes and Justin Lalor, R&D technicians at The Sherwin-Williams Company in Minneapolis, USA, for providing hands-on comments, and to James Poole, an R&D Group Leader at the same location for helpful inputs on polyester polyols.
References
- NPCS Board of Consultants & Engineers, Phenolic Resins Technology Handbook (2nd Revised Edition). New Delhi, India: NIIR Project Consultancy Services, 2019. https://www.niir.org/books/book/phenolic-resins-technology-
handbook-2nd-revised-edition-npcs-board-consultants-
engineers/isbn-9789381039977/zb,,12f,a,16,0,3e8/index.html. - K. Hirano; M. Asami, “Phenolic Resins-100 Years of Progress and their Future.” Reactive and Functional Polymers. 73 (2) 256–269 (2013). DOI: 10.1016/j.reactfunctpolym.
2012.07.003. - Geoffrey, M. M., “Foundry Binder of Phenolic Resole Resin, Polyisocyanate and Epoxy Resin.” US Patent 5,733,952, 1998.
- Wicks, Z. W. Jr., Jones, F. N., Pappas, S. P., and Wicks, D. A., Organic Coatings: Science & Technology (3rd Edition). Hoboken, New Jersey: John Wiley & Sons, Inc., 2007.
- Dwivedi, R. and Rastogi, R., Epoxy Resins and Their Curing Agents: A Review. https://www.chemarc.com/content/
article/epoxy-resins-and-their-curing-agents-a-review/
5b279cac311dbf7f7cb83cbc, (login required, accessed June 12, 2020), 2018. - Müller, B. and Poth, U. Coatings Formulation: An International Textbook (2nd revised edition), pp. 145–147. Hannover, Germany: Vincentz Network, 2011.
- Tejado, A., Kortaberria, G., Peña, C., Labidi, J., Echeverria J.M., and Mondragona, I., “Isocyanate Curing of Novolac-Type Ligno-Phenol–Formaldehyde Resins.” Industrial Crops and Products, 27 (2) 208–213 (2008). DOI: 10.1016/j.indcrop.2007.07.009.
- Avelino, F., Miranda, I. P., Moreira, T. D., Becker, H., Romero, F. B., Taniguchi, C. A. K., Mazzetto, S. E., and Souza Filho, M. S. M., “The Influence of the Structural Features of Lignin-Based Polyurethane Coatings on
Ammonium Sulfate Release: Kinetics and Thermodynamics of the Process.” J Coat Technol Res, 16 (2) 449-463 (2019). DOI: 10.1007/s11998-018-0123-y. - Heilen, W., “Main Areas of Application of Silicone and Silicone Combination Resins,” In: Heilen, W. and Herrwerth, S., Silicone Resins and Their Combinations, pp. 41-48. Hannover, Germany: Vincentz Network, 2005.
- Global Market Study on Anti-Graffiti Coatings: Beyond Fundamental Protection. https://www.persistencemarketresearch.com/market-research/anti-graffiti-coatings-
market.asp (accessed June 12, 2020), 2019. - Liu, X. “Characterization of Polymerization of Isocyanate Resins and Phenolic Resins of Different Molecular Weights Part 1: Morphology and structure analysis,” SWST 2015 International Convention, Jackson, WY, June 2015. https://www.swst.org/wp/meetings/AM15/pdfs/presentations/liu.pdf.
- Wu, H. D., Chu, P. P., and Ma, C. M., “Thermodynamic Properties of the Novolac Type Phenolic Resins Blended with Poly (Hydroxyl Ether of Bisphenol A).” Polymer, 39 (3) 703-709 (1998). DOI: 10.1016/S0032-3861(97)00320-0.
- Takeichi, T. and Agag, T., “High Performance Polybenzoxazines as Novel Thermosets.” High Performance Polymers, 18 (5) 777–797 (2006). DOI: 10.1177/0954008306068254.
- Ishida, H. Advanced and Emerging Polybenzoxazine Science and Technology, pp.1-222. Oxford: Elsevier, 2017.
- Ishida, H. and Agag, T., Handbook Of Benzoxazine Resins, pp. 1-183. Oxford: Elsevier, 2011.
- Kumar, K. S. S. and Nair, C. P. R., “Polybenzoxazine–New Generation Phenolics,” In: Dodiuk, H. and S. H. Goodman, Handbook of Thermoset Plastics (3rd edition), pp: 45-74 Amsterdam: William Andrew–Elsevier, 2014. DOI: 10.1016/B978-1-4557-3107-7.00003-8.
- Nair, C. P. R., “Advances in Addition-Cure Phenolic Resins.” Progress in Polymer Science, 29 401-498, (2004). DOI: 10.1016/j.progpolymsci.2004.01.004.
- Chutayothin, P. and Ishida, H. “ChutayothinCationic Ring-
Opening Polymerization of 1,3-Benzoxazines: Mechanistic Study Using Model Compounds.” Macromolecules, 43 4562-4572 (2010). - Rimdusit, S., Jubsilp, C., and Tiptipakorn, S., “Chapter 2: Polybenzoxazine Alloys,” In: Alloys and Composites of Polybenzoxazines, Engineering Materials, pp. 29-46. Singapore: Springer, 2013. DOI: 10.1007/978-981-4451-76-5_2.
- Kirschbaum, S. Landfester, K. and Taden, A., “Synthesis and Thermal Curing of Benzoxazine Functionalized Polyurethanes,” Macromolecules. 48 (12) 3811-3816, 2015. DOI: 10.1021/acs.macromol.5b00954.
- Kimura, H., Matsumoto, A., and Ohtsuka, K., “New Type of Phenolic Resin: Curing Reaction of Phenol-Novolac Based Benzoxazine with Bisoxazoline or Epoxy Resin Using Latent Curing Agent and the Properties of the Cured Resin,” J. Appl. Polym. Sci, 112 (3) 1762-1770, 2009. DOI: 10.1002/app.29301.
- Pintaude, G., “Chapter 7: Introduction of the ratio of hardness and the reduced elastic modulus for abrasion,” In: Gegner, J. (ed.), Tribology-Fundamentals and Advancements, pp. 217-231. Intech, 2013. DOI: 10.5772/55470.
- Gorodisher, I. , Sanlikov, D., and Caruso, M. M., “Amine/Epoxy Curing of Benzoxazines.” US Patent 9695273 B2, 2017.
- Yu, C. X., “Study on Open Loop Curing Temperature Reduction and Heat Resistance of Benzoxazines,” Chemical Engineering Transactions, 71 907-912 2018. DOI: 10/3303/CET1871 152.
CoatingsTech | Vol. 17, No. 7 | July 2020
