By Goliath Beniah, Linqian Feng, Jeffrey Clauson, Abraham Boateng, Hongkun He, Cameron L. Brown, and Andrew T. Detwiler, Eastman Chemical Company
Increasing awareness of environmental, health, and food safety issues, as well as increasing regulations on substances with potential health effects, are major driving forces for consumer behavioral changes. These behavioral changes propel the shift toward development of alternative products free from substances of concern. One such substance is Bisphenol-A (BPA), a chemical commonly found in the protective linings of food or beverage metal cans. BPA has undoubtedly become a major cause of concern, prompting serious examinations from various regulatory bodies across the world connecting BPA to adverse health effects.1-5
Since 2015, France has issued a ban on BPA in all packaging, containers, and utensils intended to come into contact with food.1 As recently as 2018, the European Union published a regulation that further restricts the presence or use of BPA-containing substances in plastic food contact materials. The new regulation reduces the specific migration limit for BPA in varnishes and coatings for food contact applications by an order of magnitude (from 0.6 mg/kg to 0.05 mg/kg) over previous regulations.2,3 Although the U.S. Food and Drug Administration has not issued a complete ban on the use of BPA in food can lining applications, several states such as Maryland, Connecticut, and California have placed further restrictions on its presence in food cans. Given the increasing regulatory pressure, the demand for Bisphenol-A Non-Intent (BPA-NI) metal packaging coatings will continue to increase in years to come.4
Resin manufacturers, can coating formulators, can makers, and brand owners are responding to this shifting market demand by actively innovating alternative solutions that can meet or exceed the performance of BPA epoxy coatings while minimizing the cost of these transitions. The performance requirements that BPA-NI can linings need to satisfy are high. The coatings must exhibit enough flexibility and adhesion to withstand the rigorous mechanical demands of can forming processes. They also need to survive high temperatures and high-pressure food sterilization processes in the presence of hydrolytic and corrosive environments such as low pH, acids, sulfur, and salts. Not only does the coating need to meet these high requirements pertaining to can forming and sterilization processes, it must also survive the storage test or pack test as part of the routine assessment of long-term shelf stability.6,7
Polyester coatings are one of the most promising BPA-NI alternatives to BPA epoxy-based coatings. The modularity of building blocks in polyesters allow for fine-tuning of its compositions with a wide variety of monomers, enabling the achievement of balanced properties including high-glass transition temperature, flexibility, and toughness. Several notable monomers, namely 2,2,4,4-tetramethyl-1,3-cyclobutanediol (TMCD), 1,4-cyclohexanedimethanol (CHDM), isosorbide, and tricyclodecane dimethanol (TCDDM), have emerged as promising building blocks for superior polyester performance. In particular, TMCD is known to impart a variety of excellent properties such as good temperature resistance, toughness, chemical resistance, and hydrolytic stability. TMCD is used in BPA-free specialty plastics prevalent in sports bottles. TMCD-containing protective resin systems have demonstrated good resistance to corrosion and chemical attack while simultaneously demonstrating good flexibility and adhesion in a variety of applications, including metal packaging applications.7,8 New product qualification in storage or pack tests is a vital step in the development and evaluation process of new metal packaging coatings. Pack tests may last from several months to several years and may involve filling and storing goods in cans at elevated temperatures. At specified time intervals, cans are opened and visually inspected for defects. Food and beverage cans come in various sizes and shapes, and coatings formulations vary across the industry. As the combination of can types and food products are numerous, this coating qualification effort can take significant time and financial investment, as well as collaboration across value chains. Given the cost, complexity, and time involved in qualifying new coatings, reducing qualification time has the potential to accelerate development cycles. A predictive analytical technique that can reduce the need for time-consuming pack tests would be highly desirable across the metal packaging value chain. Though the goal seems lofty, it is in the best interest of the metal packaging industry to develop techniques that may predict long-term performance for new coatings.
Electrochemical impedance spectroscopy (EIS) is a useful analytical technique that has been widely employed for numerous applications in industry and academia over several decades. This technique assesses the barrier properties of organic coatings and their time-dependent, long-term durability and performance in response to various experimental conditions.9-22 EIS can reveal a wealth of information on the state of corrosion in coating prior to the visual appearance of corrosion. With EIS, a small sinusoidal alternating current (AC) wave is applied and the resulting resistance (impedance) to current flow is assessed as a function of frequency. Impedance data are collected and analyzed with relevant electrical circuit models to understand the corrosion state of the coating. Kern and colleagues employed EIS to study the performance of cans coated with various formulas and filled with different food contents. They used direct current (DC) treatments and electrolyte aging treatments to understand and predict corrosion during storage.9 Charge transfer capacitance, related to the delaminated metal area, and electrolyte aging, respectively, were found to be reliable parameters and procedures for quality evaluation and performance ranking of various cans. De Vooys and colleagues also employed EIS as a screening tool to evaluate the suitability of coated metal packaging cans with new food products by measuring their impedance over a two-week period.10 The evolution of complex impedance at low frequency over a two-week period with threshold impedance values was used as a pass/fail criterion for product/container combinations. Others have employed EIS as a means to quantify the adhesion of different types of lacquers for food packaging,11 to measure the delamination of coatings over time when exposed to various conditions,12 to study the water and electrolyte uptake of organic coatings,13,14 and many other applications.15-22
In this study, we employed an EIS technique in conjunction with aging procedures involving concentrated food simulant and elevated temperatures for a series of coatings. The aging procedure acts to accelerate the corrosion process, while EIS provides quantitative measurements that enable in-depth understanding of corrosion mechanisms. This combination enables faster coating quality evaluations and rapid selection of formulations for further evaluation. The performance evaluation from EIS is further validated using an industrially relevant enamel rater test. This work provides fundamental insight into the relative performance of polyester resins in various formulations as part of a resin development strategy.
Experimental Procedures
EMN-MP resin is a mid-molecular weight TMCD-based polyester with a number average molecular weight (Mn) in the range of 6000–8000 g/mole and Tg ~90 °C. Commercial Control resin is a non-TMCD-based, high-molecular weight resin with an Mn of > 10,000 g/mole and Tg 100–110 ºC. The two resins were formulated into white polyurethane (PU) and gold phenolic PU formulations as representative solventborne BPA-NI 3-piece food can interior lacquers. The formulation details for white PU coatings and gold phenolic PU coatings are listed in Tables 1 and 2, respectively. All resins were reduced with Aromatic 100 (A-100) solvent to 50 wt% solids. Fascat® 9102 catalyst solution was diluted with A-100 to 10% of the original supplied concentration.
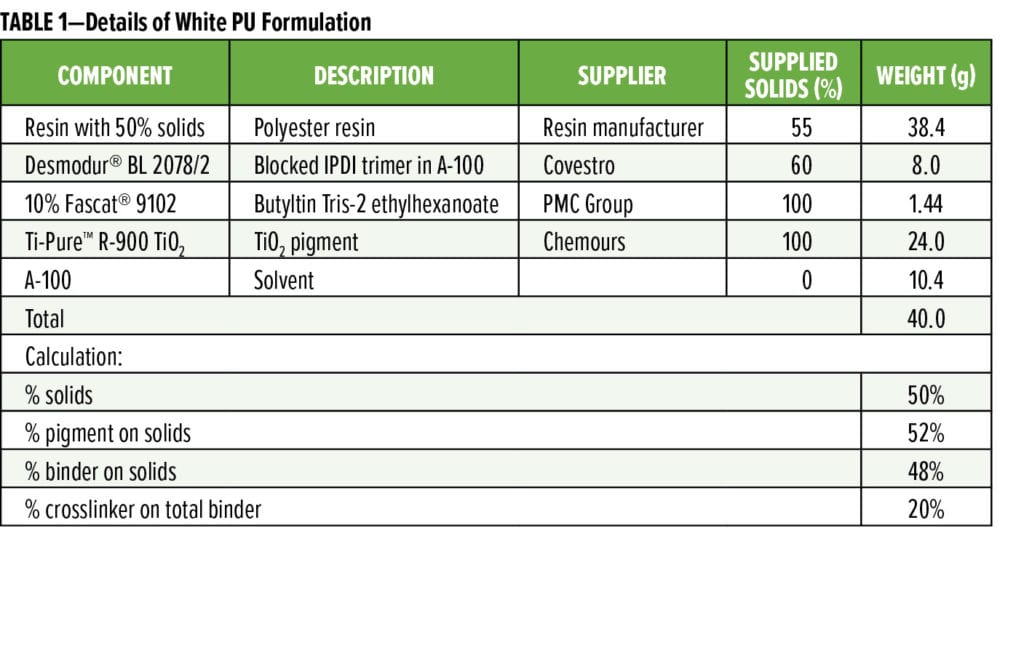
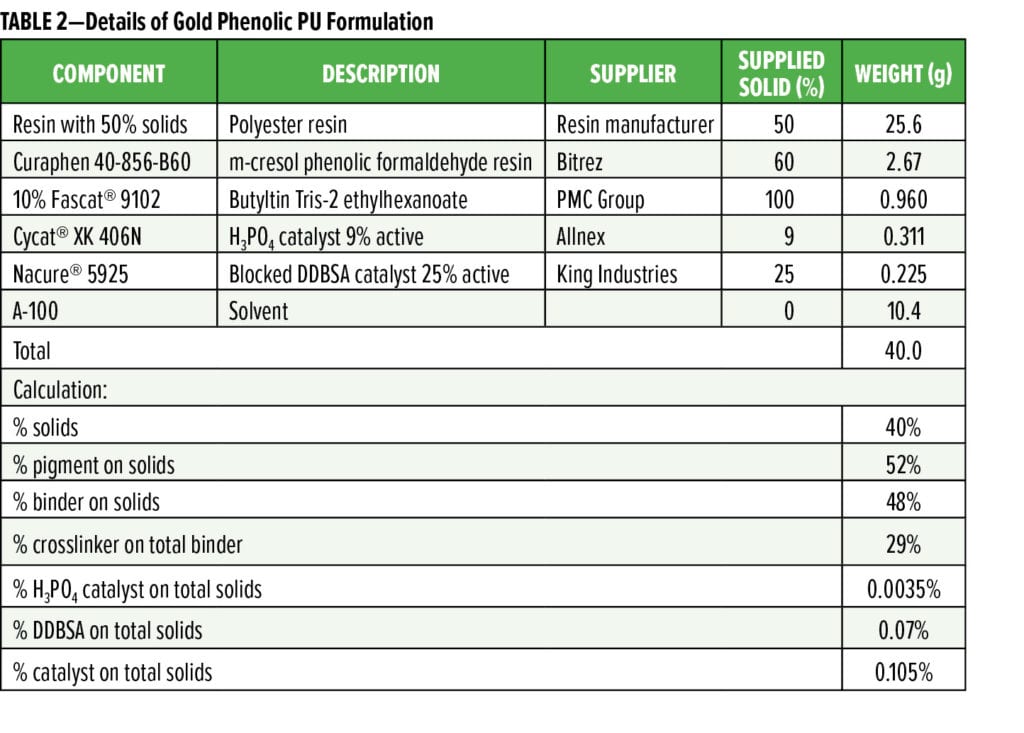
The formulations were drawn down onto electro tin plate (ETP) substrate panels supplied by Lakeside Metals Inc. (0.25# Bright T-1 0.009-0.010 x 4.0 x 12.0 in.). All formulations were applied with appropriate wire-wound rods to target film weights of 10 gram per square meter (gsm) and 6 gsm for white PU and gold phenolic isocyanate coatings, respectively. The coated panels were cured at 200 ºC for 12 min in a forced air convection oven.
Electrochemical impedance spectroscopy was performed using Gamry Instrument “Reference 600” Potentiostat equipped with Gamry framework and Echem Analyst. The samples were held in a cylindrical glass cell by a clamp fixture attached to an O-ring gasket affixed to the grooved bottom of the cell. A nickel electrode and a graphite electrode were used as reference and auxiliary electrodes, respectively. The potential was applied in a range of 5 mV from open circuit potential and the frequency was varied from 105 to 0.1 Hz.
Food simulants used in this study were 3% acetic acid (3 wt% acetic acid in 97 wt% DI water), 2% lactic acid (2 wt% lactic acid in 98 wt% DI water), and 5% acetic acid (5 wt% acetic acid, in 95 wt% DI water).
To perform EIS measurements for white PU panels, the drawn down panels were first retorted in the presence of 3% acetic acid solution. The EIS cell apparatus was fixed on top of the area of interest, and this area only was exposed to the electrolyte during the retort process at 131 ºC for one hour. Upon completion of retort and cooling, the first EIS measurements were performed, and subsequent measurements were taken at defined time points. To perform EIS measurements for gold phenolic PU panels, the area of interest in the drawn down panels was exposed to 2% lactic acid by fixing the EIS cell apparatus on top of the panels. Following 36 days of exposure to 2% lactic acid, the EIS measurements were obtained. All EIS measurements and aging were performed at room temperature. Enamel rater measurement was performed using WACO Digital Enamel Rater II. Test voltage was set at 6.3 V. Can lids were stamped from white PU flat panels and retorted in the presence of 5% acetic acid. Upon retort, the can lids were exposed to 5% acetic acid, and the can lids were placed in an oven maintained at 50 ºC. At defined time points, the can lids were taken out of oven and enamel rating was obtained.
Results and Discussion
The electrochemical impedance spectroscopy technique has been employed for decades for measuring and monitoring of time-dependent changes of organic coatings.9-22 The measured impedance values are presented in Nyquist or Bode plots and fitted to relevant and appropriate electrical equivalent circuits consisting of resistive and capacitive elements in various configurations that help with the physical interpretation of the impedance data.15-17 With this technique, the penetration of electrolyte into the coating can be followed and the initiation of corrosion at the metal/coating interface can be detected. Figure 1 shows the stages of corrosion, relevant electrical circuits, and the expected shape of Bode plots at different stages of corrosion when a coated panel is exposed to electrolyte. In Stage 0, at the start of immersion, the coatings are dry and behave like a pure capacitance. The appropriate circuit configuration is that of electrolyte resistance and coating capacitance in series. The coating capacitance depends on coatings thickness. In Stage 1, the coatings begin to absorb the electrolyte until the film micropores become saturated with the electrolyte. In this phase, the coatings can be represented by a resistance and a capacitance component in parallel. As the film continuously absorbs electrolyte, the coatings capacitance increases with increasing electrolyte content, whereas the resistance component decreases until the film becomes saturated. The film affinity towards the electrolyte and crosslinking density influence the speed of electrolyte penetration in the film. Coatings capacitance can be used to determine the change in coatings porosity or electrolyte diffusion coefficient according to the Brasher-Kingsbury equation.22 In Stage 2, as the electrolyte reaches the coatings/metal interface, corrosion is initiated and blisters in the coating begin to develop. The newly formed oxidized layer can be represented using a double layer capacitance and a charge transfer resistance in parallel, or the “two time-constants” as commonly used in EIS. There might be no visible sign of corrosion at this stage, but the magnitude of the complex impedance at low frequency continues to drop. In the subsequent stage, delamination starts, and pore breakthrough occurs along with a diffusion-limited corrosion reaction as the reactant gets delivered to the surface. A Warburg impedance component is added to the equivalent circuit in this stage.
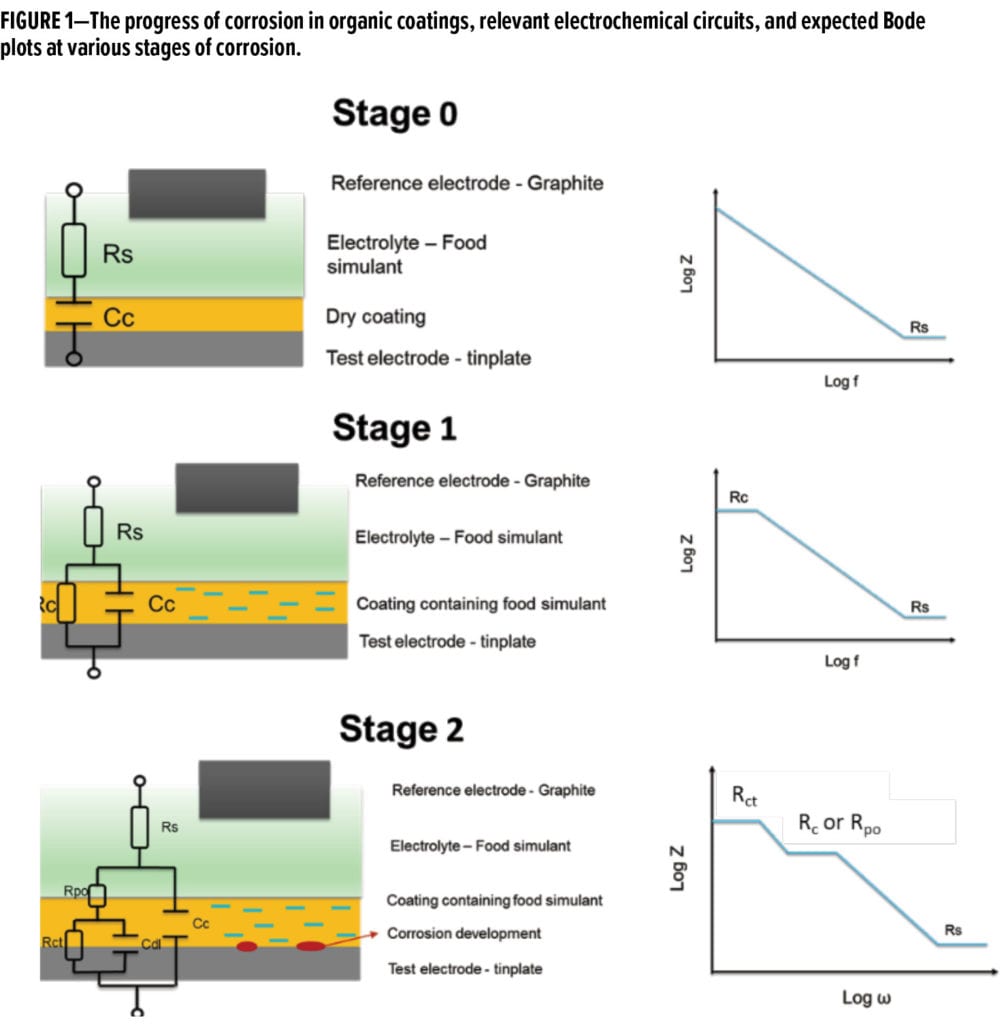
There are many ways to interpret or use the results of resistive and capacitive elements from relevant electrochemical circuits. McIntyre and Pham18 used Rpo (pore resistance) and Cdl (double layer capacitance) terms to estimate the degree of delamination in can coatings. They also used Cc (coating capacitance) to estimate the coating porosity and correlate it with flavor scalping performance. Hu et al. used a coating capacitance term to monitor water uptake and diffusion of chlorine ions in epoxy-coated aluminum alloys in the presence of NaCl solutions.13 Kern et al. used charge transfer capacitance in a slightly more complicated circuit configuration to estimate the delaminated area after exposure to food simulants.9 De Vooys et al. simply relied on the evolution of the shape of Bode plots over time and assigned relevant electrical circuits to fit the data. The magnitude of overall impedance of the coated metals exposed to food simulant over two weeks was used as a pass/fail criterion. McIntyre and Pham also used EIS to track the low-frequency impedance over several weeks and showed that the rate of decrease in impedance has some correlation with the coating porosity.18
In this study, we compare EIS responses to characterize the progress of corrosion as a proxy for long-term performance in a pack test. The magnitude of impedance at low frequency (0.1 Hz) is used to compare the relative performance of different coatings as appropriate. EMN-MP resin and Commercial Control resin were each formulated into white PU formulations (Table 1) and drawn down on tinplate. The coatings were then subjected to a retort procedure in an autoclave at 131 ºC for one hour in the presence of 3% acetic acid in a custom-made set up that exposes only the intended area to the food simulant.
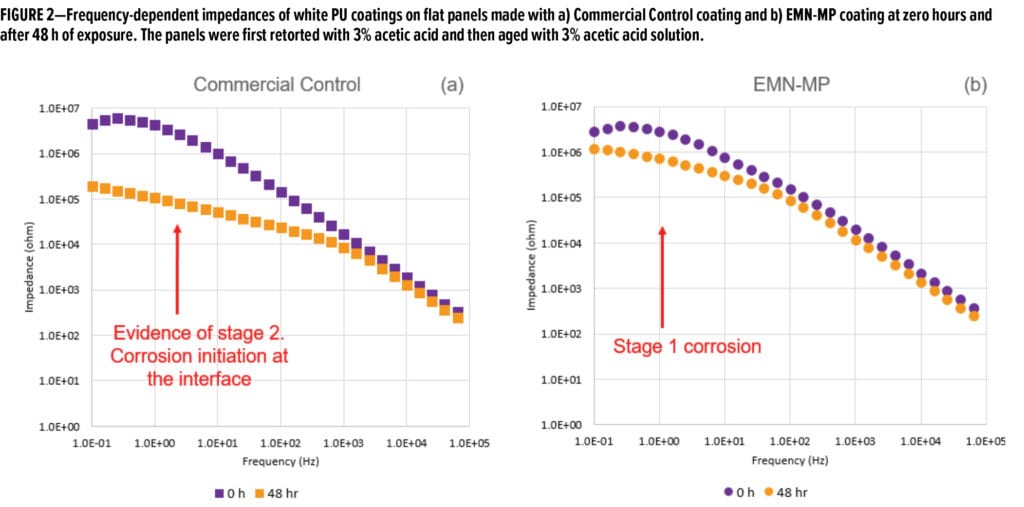
Figure 2 shows the frequency-dependent impedance values immediately after retort and after a subsequent 48 h of immersion in 3% acetic acid. At the beginning of the test, both coatings were at the same corrosion stage (Stage 1, electrolyte penetration). However, after the subsequent 48 h of exposure, the Commercial Control coating had progressed into Stage 2 corrosion, as apparent by its Bode plot shape indicating initiation of corrosion, whereas the coating based on EMN-MP resin was still in the stage of electrolyte penetration (Stage 1). This observation is supported by the general shapes of the Nyquist plots shown in Figure 3.
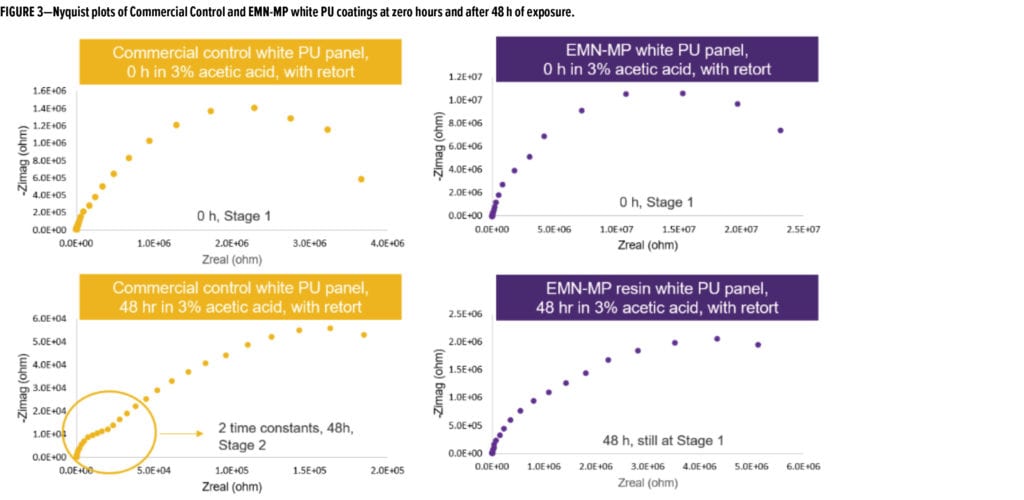
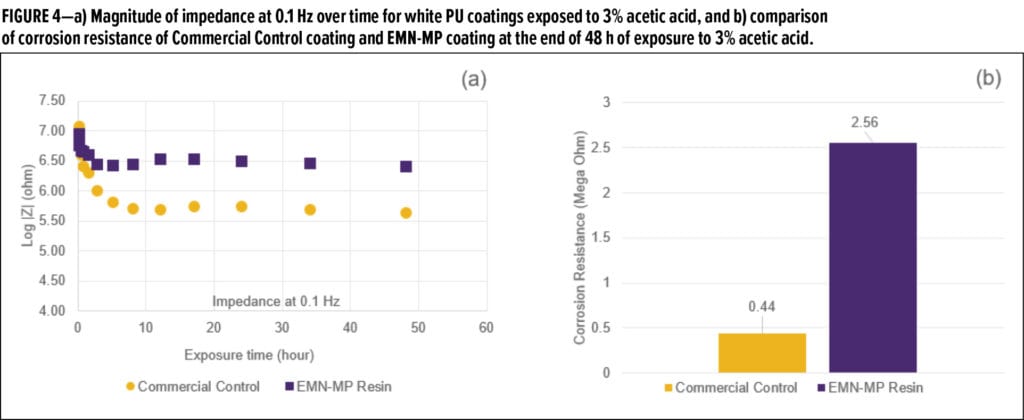
At zero hours, both coatings showed one semi-circle indicating Stage 1 corrosion. After 48 h of exposure, two semi-circles began to emerge in Nyquist plot of Commercial Control coating indicative of Stage 2 corrosion, while only a semi-circle was observed for EMN-MP coating, indicative of Stage 1 corrosion. The decrease of impedance at low frequency (0.1 Hz) for both formulas is traced in Figure 4a and consistently shows that Commercial Control coating has lower corrosion resistance over the exposure time. As the impedance values plateau at longer times, it is apparent that the corrosion slows after the initial retort exposure. EMN-MP coating demonstrates better corrosion resistance of 2.56 Mega Ohms as compared to 0.44 Mega Ohms conferred by Commercial Control coating in this post-retort plateau region (Figure 4b).
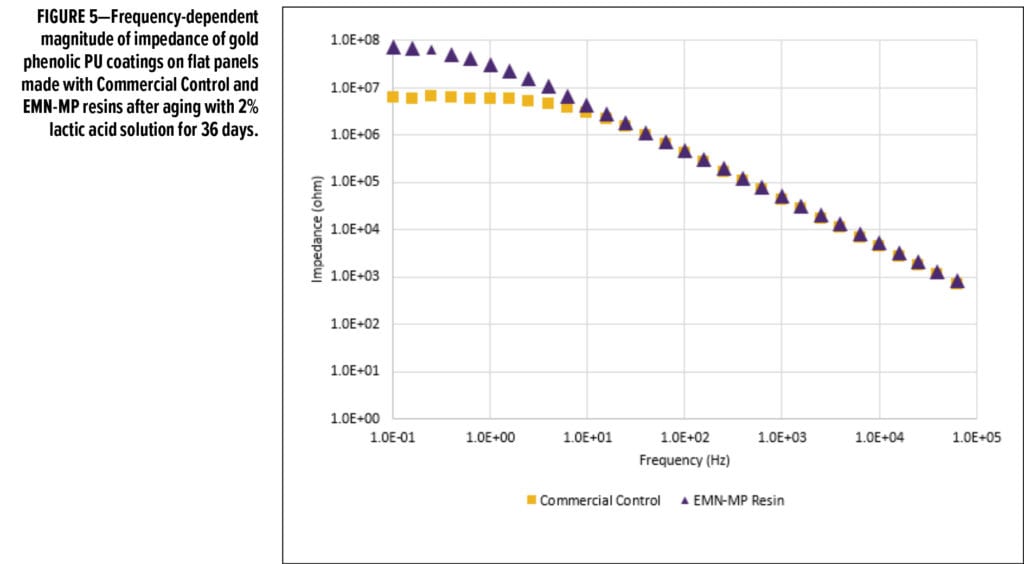
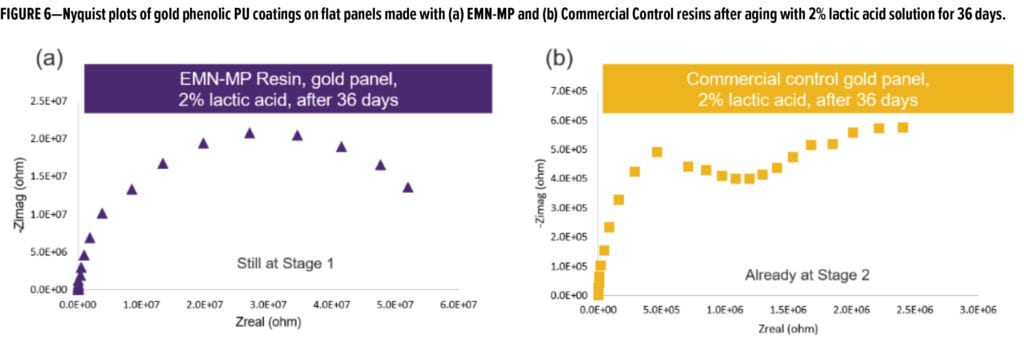
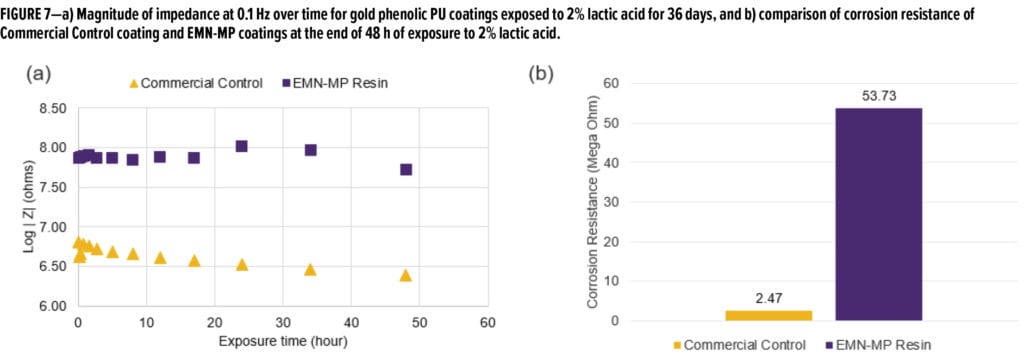
Both resins were also formulated into gold phenolic PU formulations (Table 2) and drawn down onto tin plates. Upon curing, the panels were exposed/soaked in 2% lactic acid solution and aged for 36 days at room temperature. Figure 5 shows the frequency-dependent impedance measurement of both panels after 36 days of exposure in 2% lactic acid. Figure 6 compares the Nyquist plots of both panels at the end of 36 days of exposure in 2% lactic acid. Comparing the curve shape from the Nyquist plots, it is evident that EMN-MP coating was still in Stage 1 corrosion as indicated by one semi-circle, whereas Commercial Control coating has entered into Stage 2 corrosion as evidenced from the appearance of two semi-circles. The low frequency (0.1 Hz) impedance values over 48 h of observation are shown in Figure 7a, and the corrosion resistance of both coatings are compared in Figure 7b. Again, the EMN-MP coating exhibited significantly better corrosion resistance of 53.7 Mega Ohms as compared to 2.47 Mega Ohms with Commercial Control coating in gold, phenolic isocyanate formulation at the same dry film thickness. Perhaps the higher hydrophobicity of EMN-MP resin and, therefore, its lower propensity to absorb 2% lactic acid food simulant, might contribute to the delay in corrosion development. The superior corrosion resistance observed with EMN-MP resin can be, at least in part, attributed to the hydrophobic contribution of TMCD. As evident from the two examples above, EIS techniques coupled with different aging procedures can produce quantitative and qualitative assessments that guide the development and evaluation of resin and coating performance.
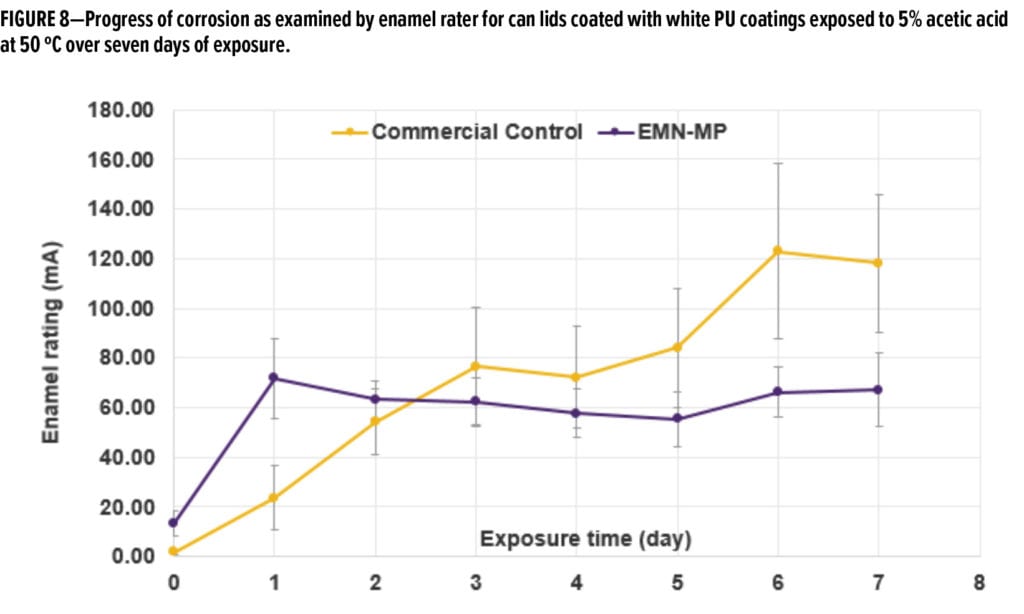
To validate the EIS results, we also compare the performance of each of these resins with enamel rater testing commonly used in industry, as documented in various patents and articles.23-27 The test included an accelerated aging approach using 5% acetic acid (2% higher than the concentration of acetic acid used for EIS experiment on white coatings) at 50 ºC over several days of exposure. This experiment was performed on stamped can lids, a notably harsher mechanical treatment than flat metal panels. It was expected that the high concentration of simulant and higher temperature would accelerate the corrosion process. Enamel rater is a DC-based technique that applies a constant 6.3-volt potential across the coating during four seconds and measures the resulting current. The enamel rating is an index of the amount of metal exposed or delaminated from the coating during exposure to food simulant. A higher current flow indicates a worse coating performance. Figure 8 tracks the current flow of can lids coated with white PU coatings exposed to 5% acetic acid at 50 ºC over seven days. The coating based on EMN-MP resin demonstrates a better corrosion resistance than Commercial Control coating at the end of seven days of exposure as evidenced by its lower current flow relative to that of Commercial Control coating.
At the end of the experiment, the exposed can lids were subjected to a tape-pull test for qualitative assessment of coating adhesion. Figure 9 shows the pictures of stamped can lids based at the seven-day exposure interval, before and after the tape-pull test. It is evident that the coating based on EMN-MP resin exhibits a qualitatively better coatings adhesion at the end of this accelerated aging test. It is also worth noting that signs of underfilm corrosion were already visible in the case of Commercial Control even prior to the tape-pull test. The tape-pull test further revealed underfilm corrosion in Commercial Control case. Signs of underfilm corrosion were also present in the exposed surface of EMN-MP coating after the tape-pull test, but the exposed area is qualitatively smaller than in Commercial Control coating, owing to better coating adhesion although quantitative assessment was not performed. The results observed in the enamel rating test are, thus, in agreement with the results of the EIS evaluation.

Conclusions
Electrochemical impedance spectroscopy is employed as a screening tool in the innovation process of new BPA-NI metal packaging resins. The resins in this study were formulated into coatings, which were subjected to aging treatments with several food simulants at relatively high concentrations. Time-dependent impedance measurements by EIS reveal the progress of corrosion in the coatings and provide quantitative measurements of corrosion resistance. EMN-MP resin in white PU coatings exhibited better barrier properties with slower corrosion kinetics and higher corrosion resistance relative to Commercial Control resin in white PU formulation. Similarly, in the gold phenolic PU formulations EMN-MP coating exhibits better barrier properties relative to Commercial Control coating. The EMN-MP resin system also shows better barrier properties in the enamel rater test on white PU coatings. The results from the enamel rater test are consistent with results observed with EIS. This work demonstrates the utility of EIS and the more commonly available enamel rater tests as powerful tools in understanding the barrier properties and corrosion kinetics of coatings as part of a resin design strategy. The data provided by these techniques can help to enable quantitative data-based decision making and faster coatings development cycles.
References
- Geueke, B. “France Bans BPA,” Food Packaging Forum (website), January 6, 2015, https://www.
foodpackagingforum.org/news/france-bans-bpa (accessed July 22, 2020). - Commission Regulation (EU) 2018,213 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending regulations (EU) No 10/2011.
- “BPA Rules in European Union Now in Force; Limit Strengthened 12-Fold,” Food Safety News (online), September 16, 2018, https://www.foodsafetynews.com/2018/09/bpa-rules-in-european-union-now-
in-force-limit-strengthened-12-fold/ (accessed July 22, 2020). - Geller, S. and Lunder, S. “BPA in Canned Food: Behind the Brand Curtain,” Environmental Working Group (website), June 3, 2015, https://www.ewg.org/research/bpa-canned-food (accessed July 22, 2020).
- Bomgardner, M. “How a New Epoxy Could Boot BPA from Cans,” Chemical & Engineering News (online), March 5, 2019, https://cen.acs.org/business/consumer-products/new-epoxy-boot-BPA-cans/97/i10 (accessed July 22, 2020).
- Geueke, B. “Good Packaging and Health: Can Coatings,” Food Packaging Forum (website), December 15, 2016, https://www.foodpackagingforum.org/food-packaging-health/can-coatings (accessed July 22, 2020).
- Kelsey, R., Scardino, B.M., Grebowicz J.S., and Chuah, H.H. “High Impact, Amorphous Terephthalate Copolyesters of Rigid 2,2,4,4-Tetramethyl-1,3-
cyclobutanediol with Flexible Diols,” Macromolecules, 33, 5810-5818 (2000). - Zhang, M., Moore, R.B., and Long, T.E. “Melt Transesterification and Characterization of Segmented Block Copolyesters Containing 2,2,4,4-Tetramethyl-1,3-
Cyclobutanediol,” J. Polym. Sci. Part A: Polym. Chem., 50, 3710-3718 (2012). - Kern, P., Baner, A.L., and Lange, J. “Electrochemical impedance spectroscopy as a tool for inestigating
the quality and performance of coated food cans,”
J. Coat. Tech, 71, 67-74 (1999). - De Vooys, A.C.A., Boelen, B., and Van der Weijde, D.H. “Screening of Coated Metal Packaging Cans using EIS,” Prog. Org. Coat, 73, 202-210 (2012).
- Barilli, F., Fragni, R., Gelati, S., and Montanari, A. “Study on the Adhesion of Different Types of Lacquers Used in Food Packaging,” Prog. Org. Coat., 46, 91-96 (2003).
- Mahdavi, F., Tan, M.Y.J., and Forsyth, M. “Electrochemical Impedance Spectroscopy as a Tool to Measure Cathodic Disbondment on Coated Steel Surfaces: Capabilities and Limitations,” Prog. Org. Coat., 88, 23-31 (2015).
- Hu, J.M., Zhang, J.Q., and Cao, C.N. “Determination of Water Uptake and Diffusion of Cl- Ion in Epoxy Primer on Aluminum Alloys in NaCl Solution by Electrochemical Impedance Spectroscopy,” Prog. Org. Coat., 46, 273-279 (2003).
- Dornbusch, M., Kirsch, S., Henzel, C., Deschamps, C,. Overmeyer, S., Cox, K., Wiedow, M., Tromsdorf, U., Dargatz, M., and Meisenburg, U. “Characterization of the Water Uptake and Electrolyte Uptake of Organic Coatings and the Consequences by Means of Electrochemical Impedance Spectroscopy and UV-vis Spectroscopy,” Prog. Org. Coat., 2, 89, 332-343 (2015).
- Loveday, D., Peterson, P., Rodgers, B. “Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy Part 1: Fundamentals of Electrochemical Impedance Spectroscopy,” J. Coat. Tech., 8, 46-52 (2004).
- Loveday, D., Peterson, P., and Rodgers, B. “Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy Part 2: Applications of EIS to Coatings,” J. Coat. Tech., 8, 88-93 (2004).
- Loveday, D., Peterson, P., and Rodgers, B. “Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy Part 3: Protocols for Testing Coatings with EIS,” J. Coat. Tech., 2, 22-27 (2005).
- McIntyre, J.M.; Pham, H.Q. “Electrochemical Impedance Spectroscopy: A Tool for Organic Coatings Optimizations,” Prog. Org. Coat., 27, 201-207 (1996).
- Da Silva, L.R.R., Avelino, F., Diogenes, O.B.F., Sales, V. de O.F., da Silva, K.T., Araujo, W.S., Mazetto, and S.E., Lomonaco, D. “Development of BPA-free Anticorrosive Epoxy Coatings from Agroindustrial Waste,” Prog. Org. Coat., doi.org/10.1016/j.porgcoat.2019.105449.
- Romano, A.P. and Olivier, M.G. “Investigation by Electrochemical Impedance Spectroscopy of Filiform Corrosion of Electrocoated Steel Substrate,” Prog. Org. Coat., 89, 1-7 (2015).
- Amirudin, A. and Thierry, D. “Application of Electrochemical Impedance Spectroscopy to Study the Degradation of Polymer-Coated Metals,” Prog. Org. Coat., 26, 1-28 (1995).
- Brasher, D.M. and Kingsbury, A.H. J. Appl. Chem., 62, 4 (1954).
- Patel, H.K. US Patent 4,963,602. Aqueous Epoxy Resin-Acrylic Resin Coating. Compositions Containing Also Phenoxy, Novolac and Resole Resin Composition.
- Westerof, W., Kolk, D.J., Van Riggelen, W.J.P.S.M. US Patent 5,739,215. Use of a Polyester in the Preparation of Coatings for the Interior of Can Ends.
- McVay, R.L. US Patent 7,475,786. Can Coatings, Methods for Coating Can and Cans Coated Thereby.
- Heyes, P.J. US Patent 5,238,517. Production of Laminated Materials.
- Kojima, S., Watanabe, Y. “Flexibility of Epoxy Coatings. Part 2: The Relationship with the Degree of Cure,” J. Appl. Polym. Sci., 36, 224-228 (1996).
CoatingsTech | Vol. 17, No. 8 | August 2020
