By Leo Procopio, Paintology Coatings Research, LLC
The entire North American paint and coatings industry met in person for the first time in four years during the American Coatings Show and Conference, held April 5–7 in Indianapolis. The last in-person exhibit and conference were held in 2018—two years prior to the COVID-19 pandemic.
By all accounts, the 2022 conference and exhibit were overwhelming successes. People in the industry have been missing the in-person interaction for more than two years due to pandemic-related restrictions and were ready to get together to discuss all things concerning paint and coating.
The American Coatings Conference consisted of nearly 760 attendees, all engaging in 90 presentations describing the latest advances in coatings technology.
This article includes technical highlights from many of the sessions, which were held concurrently in four tracks. Further details can be found in the papers available in the proceedings of the conference. Some papers will also be published in future issues of CoatingsTech after peer review.
The papers highlighted in this article will be presented according to some overarching themes that they addressed: Sustainability, Functional Coatings, Paint Fundamentals, and Additive Technologies. These brief summaries will hopefully serve to interest the reader to seek out the full papers and learn more about the thought-provoking topics presented during the conference.
Sustainability
Sustainability and sustainable development are concepts that are front and center for every business today. While sustainability can be defined as the long-term goal, as in achieving a more sustainable world, sustainable development is the process through which we achieve that goal. One definition of sustainable development comes from Our Common Future (aka, the Brundtland Commission Report), prepared in 1987 by the World Commission on Environment and Development.1 It defines sustainable development as “development that meets the needs of the present without compromising the ability of future generations to meet their own needs.”
The concept of designing products which have lower impact on the environment and society is not new for the paint and coatings industry. Goals such as lowering volatile organic compounds (VOCs) in coatings and finding alternatives to surfactants that contain alkylphenol ethoxylates have been industry concerns for decades.
More recently, innovation efforts have focused on additional topics such as removing other materials of concern (e.g., PFAS), using biobased raw materials, and lowering carbon footprint. The impact of sustainability trends in innovation were clearly displayed at the exhibit and conference at booths, product presentations in the exhibit hall, and conference papers and presentations.
One such paper, “The use of levulinates as coalescing agents in water-based coatings,” by Steven Block of NXTLEVVEL Biochem, received the American Coatings Award for most outstanding paper. Block described how NXTLEVVEL Biochem has commercialized a line of products derived from levulinic acid, which the U.S. Department of Energy has identified as one of the top 12 renewable chemicals. The products include levulinate esters and levulinic ketals (Figure 1), and are derived from non-food biomass such as corncobs and bagasse from sugar cane.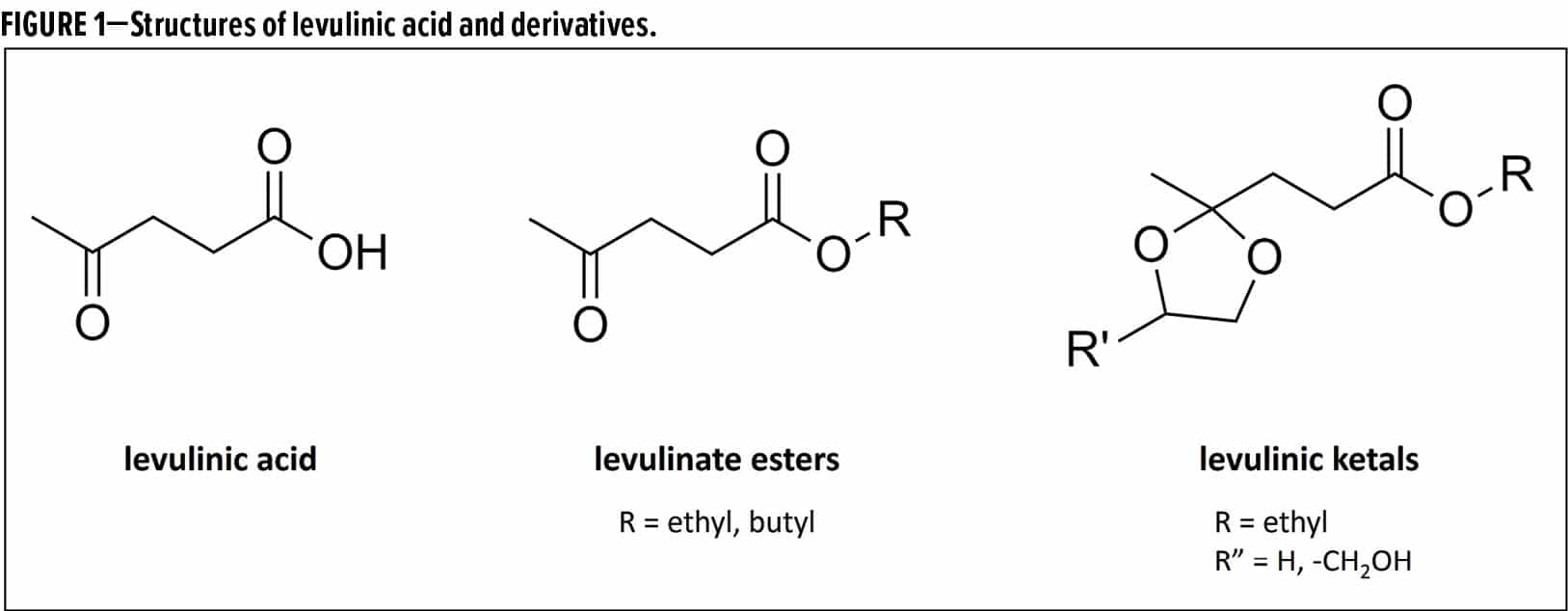
Block described how the biobased content of the derivatives could be up to 100% biobased. For example, butyl levulinate can be produced at either 58% or 100% biobased content, depending on whether or not the source of butanol is biobased.
In addition, they have a lower carbon footprint compared to petrochemical-based solvents. The example given was for ethyl levulinate (0.347 kg CO2-eq/kg) vs typical petrochemical based solvents (2 to 5 kg CO2-eq/kg). The levulinate esters (R = ethyl, butyl) are readily biodegradable, and the ketals are ultimately biodegradable.
The use of derivatives including butyl levulinate and ethyl levulinate propanediol ketal as coalescing solvents for waterborne coatings was investigated. Due to their low water solubility and slow evaporation rates, it was proposed that they could be good coalescents for latex coatings.
They were compared to some common coalescents, such as 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (aka Texanol). Evaluations in waterborne coatings based on several acrylic and acrylic/fluoropolymer emulsion polymers were conducted and demonstrated that the levulinate derivatives gave comparable or better compatibility, MFFT reduction, gloss and hardness development and water-resistance properties compared to the standard coalescing solvents. On a weight basis, the levulinate derivatives were more efficient at lowering MFFT compared to the standard solvents.
A second presentation discussing biobased raw materials was “Lignin as a raw material for production of biobased resins,” by Mojgan Nejad of Michigan State University. Nejad explained that after cellulose, lignin is the second-most abundant natural polymer, and is a byproduct of pulp and paper and bioethanol production. Lignin is a random polymer derived from phenolic precursors (p-coumaryl, coniferyl and sinapyl alcohol), and its composition varies based on the biomass source as well as the isolation method.
Because it varies so widely, Nejad stressed that analytical methods are necessary to characterize the lignin being evaluated. For example, 31P NMR is used to measure hydroxyl content (following conversion to phosphate derivatives). Molecular weight, polydispersity index, elemental analysis, and characterization of aliphatic and aromatic hydroxyls are all important information to gather.
Lignin was evaluated as a replacement for phenol in phenol-formaldehyde adhesives. A relatively low molecular weight lignin was reacted with formaldehyde to produce a lignin-formaldehyde resin. In addition, glyoxal was used as a biobased alternative to formaldehyde to generate a completely biobased lignin-glyoxal resin.
Using differential scanning calorimetry (DSC), it was found that the lignin-formaldehyde resin cured at the same temperature as a commercial phenol-formaldehyde resin, while the lignin-glyoxal resin needed slightly higher temperatures (165 °C vs 145 °C).
A reactivity analysis of 20 varieties of lignin with phenylisocynanate was done to identify an appropriately reactive lignin to replace the polyol in polyurethane formulations. The lignin polyol was used in a wood coating and compared to a commercial polyurethane.
Although there was initially some concern about color, such a lignin-based polyurethane appears to have suitable appearance over wood or could be used in applications where color is not an issue. Nejad also described replacing bisphenol A in epoxy resins by modifying lignin with biobased epichlorohydrin (from glycerol) to form an epoxidized lignin resin.
The lignin-based epoxy resin was combined with a commercially available biobased curing agent (derived from cashew nutshell liquid) to form a fully biobased epoxy system. Nejad ended her presentation with the excellent question to the audience, “Isn’t it time to start thinking about using lignin-based resins to formulate more sustainable coatings?”
In the presentation, “Aliphatic glycidyl ethers as crosslinkers for high-performance NISO coatings,” Brendon Bohnert of Nagase Specialty Materials NA discussed new epoxy crosslinkers, made from biobased sorbital, which can be used in two-component, high performance non-isocyanate (NISO) coatings. Bohnert described how there is interest in coatings made without isocyanate for EH&S reasons, but that there are also challenges in meeting the high-performance requirements.
Two tetrafunctional aliphatic epoxy crosslinkers were described, based on the epoxidation of biobased sorbitol (Figure 2). One is designed for waterborne coatings (EEW = 173 g/eq), and the other for solventborne coatings (EEW = 191 g/eq), with about 15% biobased content.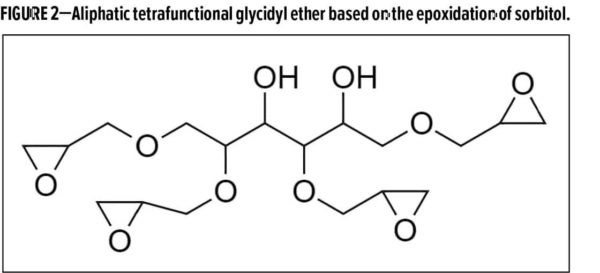
Each was evaluated as a crosslinker for acid-functional acrylic resins in formulated 2K NISO systems and tested direct-to-metal (DTM) and as a topcoat over organic zinc-rich and epoxy primers. Comparisons were made to a 2K solventborne acrylic polyurethane and a commercial 2K solventborne NISO topcoat.
The solventborne aliphatic epoxy crosslinker gave an ambient-cure NISO system with promising corrosion resistance and durability (gloss and color retention in accelerated Xenon arc testing) and improved flexibility/impact resistance compared to the commercial NISO and 2K polyurethane topcoats.
Bohnert added that formulating work will continue, aimed at improving pot life and dry time and optimizing corrosion resistance. The waterborne NISO system displayed excellent pot life, dry time and impact resistance, and Bohnert alluded to further formulating work designed to improve gloss and corrosion resistance.
The last presentation with a sustainability theme to be described here was “Improving the odds of success using a benign-by-design approach to product development,” presented by Ingrid Meier of Evonik Corporation.
Meier discussed a unique strategy used in the development of new dynamic wetting agents. Dynamic wetting agents require molecules to quickly align at the air/water interface, especially after the system is disturbed. Key structural features include short, often branched hydrophobes, which prevent strong micelle formation.
Dynamic wetting agents are also low molecular weight and can’t meet the U.S. Environmental Protection Agency (EPA) definition of a low-risk polymer under the Toxic Substances Control Act (TSCA), which in turn brings considerable risk to the registration process for a new molecule due to the significant time and cost for required testing.
EPA uses quantitative structure-activity relationships to model potential ecotoxicity behavior, and has a publicly available model (EPI Suite™) that can be downloaded from its website and used to understand how the EPA might view the risk profile of a new substance in absence of data.
The project team utilized the modeling to predict the ecotoxicity of “paper” molecules, and along with their understanding of structural requirements for dynamic wetting and low foam, drive the development process and minimize the amount of ecotoxicity testing needed.
In a pre-concept stage, the team identified nine chemical classes which they predicted to perform well as low-foam dynamic wetting agents. Using the models in EPI-Suite to predict aquatic ecotoxicity and biodegradability, they were able to narrow the choices to three chemical classes: thioethers, hydroxythioethers, and N,N-dialkylglucamines.
Examples were prepared and then all components present at > 1% were identified, and EPI Suite was used to predict their environmental, health, and safety profile. Meier explained that after further fine tuning for performance, the two leading candidates created by the benign-by-design process, a thioether and hxdroxythioether, were ushered through the registration process quickly and efficiently.
Functional Coatings
Another key trend in coatings technology has been the development of coatings with functionality beyond the typical protective and aesthetic properties of paints and coatings. For example, functional coatings that provide enhanced haptics, self-healing, thermal insulation, and anti-microbial properties are available. The topic of functional coatings was well represented at the conference, with a full-day session devoted to the subject.
A discussion on coatings capable of removing formaldehyde from the air in a building was presented by Mark Langille of Angus Chemical Company in the presentation, “Creating functional coatings with formaldehyde-scavenging additives.”
Formaldehyde has been identified as a key chemical species that negatively affects indoor air quality, and which has detrimental effects on human health. As Langille explained, there are many sources of formaldehyde, such as cigarette smoke, particle board used in furniture, and carpeting.
A French study was cited that showed that formaldehyde is a high concentration contaminant of indoor air, and the World Health Organization has set a threshold where a formaldehyde level above 80 ppb (100ug/m3) is considered contaminated air.
Tris(hydroxymethyl)aminomethane (TRIS AMINO™), an aminoalcohol additive, was evaluated as an additive for paints and coatings which have the ability to react with airborne formaldehyde and remove it as an indoor air contaminant.
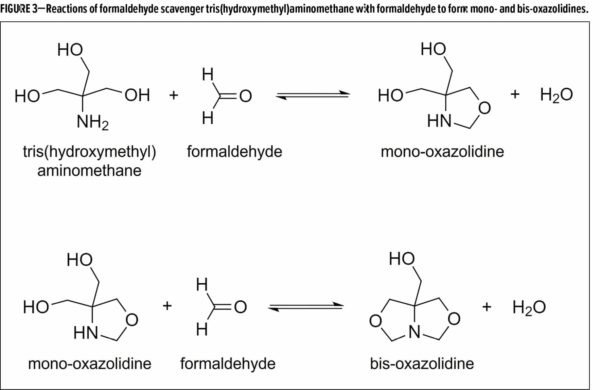 The reaction of TRIS-AMINO with two equivalents of formaldehyde proceeds through a mono-oxazolidine to eventually produce a bis-oxazolidine and water (Figure 3). Although the reactions are reversible, harsh conditions (i.e., acid) are needed to regenerate the starting materials, and under ordinary circumstances the oxazolidine products are quite stable.
The reaction of TRIS-AMINO with two equivalents of formaldehyde proceeds through a mono-oxazolidine to eventually produce a bis-oxazolidine and water (Figure 3). Although the reactions are reversible, harsh conditions (i.e., acid) are needed to regenerate the starting materials, and under ordinary circumstances the oxazolidine products are quite stable.
TRIS AMINO was incorporated into a model architectural paint formulation at levels of 0 to 0.8% on total formulation weight, and dried paint films were evaluated according to the method of ISO 16000-23 to demonstrate the ability to remove formaldehyde.
Coated panels were placed in a chamber and air containing formaldehyde (at 80 ppb) was circulated through the chamber, and formaldehyde concentration was measured for air exiting the chamber.
Without a scavenger, only 0 to 10% of the formaldehyde is removed from the air after 21 days, while approximately 50% is removed by the sample containing TRIS AMINO. Other paint properties were similar to the control with no additive.
Andrew Bartlett of Chromaflo Technologies examined bringing the functionality of electrical conductivity to coatings in the presentation, “The use of single-walled carbon nanotubes in coatings colorants.”
Nanoparticles can be difficult to handle and disperse in a manufacturing environment, and Bartlett explained how Chromaflo Technologies has created single-walled carbon nanotube (SWCNT) dispersions to take the potential issues of handling and dispersing SWCNTs out of the paint manufacturer’s facilities. The SWCNT dispersions are already used in thermoset applications, and studies were initiated to understand their potential utility in paints and coatings.
For waterborne coatings, the addition of SWCNTs to phthalo green and bismuth vanadate colorants was investigated, with levels of 0, 40, 400, and 657 ppm for the phthalo green colorant, and 0, 40, and 400 ppm for the bismuth vanadate colorant.
Coatings with electrical resistance in the range to allow static dissipation could be achieved at 400 ppm and above, but electrical resistance was not quite low enough for the coatings to be considered conductive.
Color is sacrificed at the higher levels of SWCNT, i.e., the color becomes darker. However, a comparison with carbon black at the same 400 ppm in the yellow bismuth vanadate colorant shows that the carbon black causes much more darkening compared to the SWCNT.
In a solventborne acrylic clear base, addition of 400 ppm SWCNT achieved conductivity, but was lost on addition of phthalo green colorant. Bartlett proposed a few possible reasons why the clear base with only SWCNT was conductive while the pigmented base was not, including interactions of dispersants with the SWCNT, steric interference of the SWCNT conductive pathway by the pigments, and possible affinity of the SWCNT for the pigment surface.
Further study with inorganic pigments (red iron oxide and TiO2 ) and an alternative additive package demonstrated the ability for pigmented bases to remain conductive.
Overall, Bartlett stressed that these were early studies, and more work needs to be done. However, the initial results show promise that color spaces with conductivity can be achieved by using SWCNT dispersions.
In another interesting presentation on functional coatings, a unique anti-microbial additive was discussed by Avantika Golas of Corning Incorporated. The paper, “Key considerations for functional virucidal paints,” described the use on a novel copper-glass ceramic additive as a sustainable delivery system for Cu+1 ions with high anti-microbial efficacy.
Golas first described how surfaces facilitate the spread of microbes through both indirect contact (touching a previously contaminated surface) and aerosol spread, and that spread can be very fast. As an example, it has been shown that a contaminated surface can lead to spread throughout a building within 2 to 4 hours.
Disinfection techniques, such as liquid spray, are prone to errors (surfaces missed during cleaning) and are episodic in nature, while contamination occurs constantly. Therefore, surfaces with residual efficacy can provide an additional layer of protection.
Golas noted how testing of products claiming to be bactericidal and virucidal needs to be conducted to simulate realistic environmental conditions and contamination. Wet testing has strong incumbency, and uses test conditions at elevated temperature (37 °C) and humidity (>95% RH).
However, there is a slow trend towards regulatory adoption of dry test standards, which are done at room temperature (20-24 °C) and ambient humidity (40-50% RH). To pass the dry test conditions, a truly bactericidal/virucidal product must demonstrate a > 3 log kill of microbes on the surface within 2 hours, i.e., greater than 99.9% reduction in the number of microbes remaining.
The study compared interior latex paints containing copper-glass ceramic particle with anti-microbial coatings based on other additives. Efficacy of the paint with copper-glass ceramic particles was better in both the dry and wet test methods.
Confocal fluorescence microscopy was used to visualize the live/dead bacteria after testing and showed significant bacterial death on the coating with copper-glass ceramic additives. In comparison, a silver-based antimicrobial coating continued to show live bacteria, similar to an inert stainless steel surface.
Dry testing using bacterial cultures (Staphylococcus aureas) showed greater than 99.99% reduction on the paint with copper-glass ceramics, equivalent to a bare copper surface. Coatings containing silver or quaternary ammonium additives had considerably worse performance (< 1.5 log reduction).
Golas also explained that cross-contamination can occur quickly, and that the kinetics of killing microbes is meaningful. Experiments with SARS-CoV-2 virus show > 3 log reduction in only 30 minutes with the coating based on copper-glass ceramic particles, and complete reduction within 60 minutes.
Paint Fundamentals
Development of coatings with improved performance requires an understanding of the basic processes that drive properties. Examples of some processes important to coatings technology include wetting, rheology, dispersion, hiding, and durability. Several conference papers dealt with studies that provide a better understanding of paint fundamentals, including two highlighted below.
Michael Diebold of Chemours presented an interesting paper discussing the role of titanium dioxide in coating durability in the presentation, “Separating the effects of TiO2 dispersion and photoactivity on paint durability.”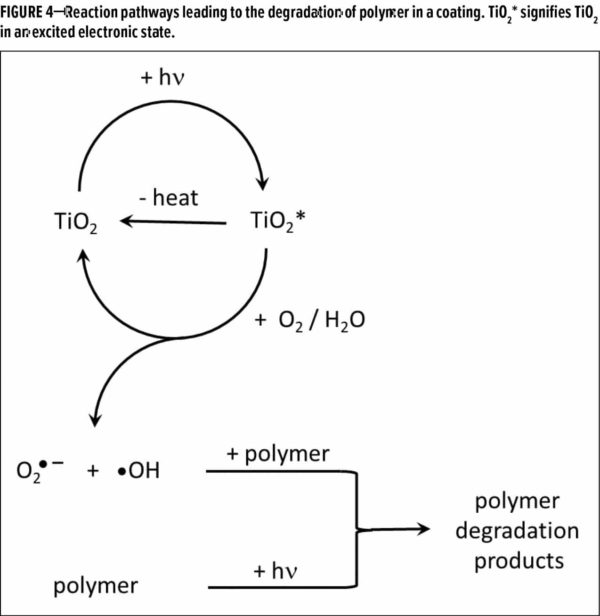
Titanium dioxide (TiO2 ) is a key pigment used in paints and coatings, and as Diebold explained, it affects coating durability in multiple ways. By itself, TiO2 is very durable, and coatings degrade (i.e., lose gloss, color fade, and chalk) due to breakdown of the polymeric binder, not because of degradation of the TiO2. One mechanism for polymer degradation involves ultraviolet (UV) light directly interacting with the resin, breaking bonds to generate radicals, and leading to degradation products (Figure 4).
Because it is a strong absorber of UV light, TiO2 can protect underlying resin by converting UV light to harmless heat energy via an excited state intermediate. In this role, TiO2 effectively casts a shadow on the resin beneath and protects it from direct photolysis by the UV light.
However, in the presence of water and oxygen, the energy which TiO2 absorbs from UV light is sometimes converted to chemical energy by generating superoxide and hydroxyl radicals, which can then break down the organic binder. TiO2 acts as a catalyst in both pathways (Figure 4), and the surface is typically treated with a layer of silica or alumina to minimize the effect of the photocatalytic activity on polymer degradation.
Diebold explored the role of TiO2 dispersion on coating durability, in particular on the effect of color fade. Previous work has established that initial gloss, which Diebold described as a proxy for pigment dispersion, has an effect on gloss retention that is independent of the photocatalytic activity.
In a paint with poor TiO2 dispersion, for example, as polymer degrades from the surface, pigment agglomerates are exposed that roughens the surface and lowers gloss more quickly than for a well-dispersed sample.
The effect of pigment dispersion on color fade (chalking) has not been studied in the same manner, partly because paints with poor TiO2 dispersion also have poor opacity and hiding, and therefore are not of interest to investigate further with weathering studies which require time and money.
In the study, Diebold evaluated a series of TiO2 pigments prepared with different surface treatments, i.e., varying levels of photocatalysis inhibitor and dispersion enhancing agent. The TiO2 samples were formulated into architectural coatings tinted with blue colorant. Initial gloss and initial brightness (L*) were found to correlate well with one another, and thus initial L* was used as the proxy for degree of TiO2 dispersion.
Initial L* values confirmed a lower degree of dispersion for most of the experimental samples compared to several commercial grades of TiO2. Coatings were exposed in an accelerated outdoor exposure, and durability measured as the change in color (delta E).
Diebold’s convincing argument is that the contributions of TiO2 photocatalytic activity and dispersion on durability can be separated and quantified. A full explanation of the analysis is too detailed for this summary, but his conclusion is that the effect of the degree of TiO2 dispersion on durability can swamp out the effect of TiO2 photocatalytic activity. Therefore, improving TiO2 dispersion, rather than decreasing photocatalytic activity, is a promising route to improving coating durability.
In the presentation, “Improving application experience and applied hide for professional paints via additives optimization,”Sunny Wang from Dow Coating Materials discussed some of the basics behind formulating for good touch-up and hiding properties.
First, an analysis of the product claims for 72 commercial paints targeted at the professionally applied interior architectural paint market was described, showing that half of the top 10 claims is related to application properties such as touch-up and hiding. Hiding/coverage, stain removal, and touch-up are the top three properties claimed.
Benchmark testing of the paints was completed, evaluating both film properties (stain and scrub resistance, contrast ratio, tint strength, adhesion, hardness, and surfactant leaching) and application properties (roller applied hiding, touch-up, surface smoothness and sag/leveling). Analysis shows that paints based on acrylic binders are more often rated higher for film properties than vinyl acrylic or VAE binders.
Wang then described studies targeted at developing a fundamental understanding of two of the important application properties, applied hiding and touch-up. Also discussed were examples of how additives such as rheology modifiers and defoamers can be used to improve applied hiding and touch-up.
Touch-up properties rely on uniformity of color, sheen, and surface pattern (i.e., topography). Wang described evaluations conducted to understand how choice of rheology modifiers, dispersants and application method can influence surface pattern and color development.
For example, paints based on various types of dispersants (polyacid, hydrophilic, hydrophobic) and rheology modifiers (HEC and HASE) showed variation in color development, with the HASE-thickened paints having more consistent color development across dispersant type.
Elemental analysis of paint surfaces showed a paint thickened with HEC had a more uneven distribution of polymer and extender pigment compared to one thickened with HASE rheology modifier, suggesting a reason for the variation in color properties.
Applied hiding was described as being dependent on three factors: intrinsic hiding due to opacifying pigments in the formulation which scatter light, film build, and pattern uniformity. Wang discussed how pattern uniformity in particular can affect applied hiding.
For example, paints applied by brush or roller often have thick and thin spots due to the surface pattern. In theory, the paint loses more hiding due to low/thin spots compared to what it gains from high/thick spots, so a rough, uneven surface pattern leads to worse overall applied hiding.
Wang then provided examples of how optimizing the rheology modifier package to balance sag resistance and flow/leveling can provide excellent pattern uniformity and better applied hiding.
Additive Technologies
Although they make up a relatively small percentage of a coating by weight, additives often carry a heavy load when it comes to ensuring good performance. While the choice of binder and pigment are obviously critical factors in a paint formulation, the proper selection of additives can have a strong effect on properties such as wetting, rheology, block resistance, film formation, foam control, and water and corrosion resistance, among many others.
It is only fitting that a number of the conference presentations focused on new additive technologies. Some have already been discussed above, but a few that are focused on new surfactant technologies will be described here.
A new surfactant for the manufacture of waterborne epoxy dispersions was discussed by Lichang Zhou of Solvay in the presentation, “Reactive epoxy emulsifier for high-performance waterborne epoxy coatings.”
Waterborne epoxy coatings are finding use in many protective and general industrial finishing markets, with key drivers being environmental regulation and quickly rising demand in Asia markets. A key challenge to the waterborne technology however is perceived lower performance.
Two common methods of getting epoxy resins into waterborne forms include the use of external surfactants to emulsify the resin in a phase inversion technique (external emulsification) and modifying the resin structure to include hydrophilic groups that allow emulsification (internal emulsification). Both of these techniques incorporate hydrophilic material into the final product that can lead to performance issues.
A third technique that Zhou highlighted is the use of a reactive emulsifier. The non-ionic, APEO-free reactive emulsifier allows the formation of epoxy emulsions with good particle-size control, and excellent stability in both the emulsion and formulated coatings. Upon curing, the emulsifier becomes incorporated into the polymer matrix via crosslinking, and therefore is not as detrimental to properties as a non-reactive surfactant.
The examples described by Zhou were based on emulsification of a solid epoxy resin (EEW = 450 – 500), to give a small particle size (< 1 um) and high solids (55%) emulsion. The epoxy emulsion prepared with the reactive emulsifier was compared to commercial epoxy emulsions in both low- and high-PVC red-primer formulations. The experimental sample displayed overall excellent performance, including paint stability, corrosion resistance, metal adhesion, hardness, impact resistance, and solvent resistance.
A second paper describing the use of reactive surfactants was presented by Juliane Santos of Oxiteno. The presentation, “Improving water resistance of water-based coatings using reactive surfactants,” discussed use of a new reactive surfactant for emulsion polymerization.
Santos described how surfactants are responsible for stability of latex polymers, but often a source of poor water resistance. In the final stages of film formation, if a surfactant is not compatible with the polymer it will form pockets of surfactant in the dry film, which are then a source of poor water resistance. Incorporation of the surfactant into the polymer backbone is expected to aid in water resistance.
The team developed a new nonionic reactive surfactant, with unsaturation in the hydrophobic end of the surfactant. This allows the reactive surfactant to adsorb and pack onto the particle surface as well as a conventional surfactant, prior to its copolymerization with the monomers. After polymerization, it can stabilize the latex particle better than reactive surfactants with unsaturation in the hydrophilic portion of the surfactant.
Properties for the new reactive surfactant were demonstrated by using it to prepare styrene-acrylic latex polymers, with close to 70% of the reactive surfactant incorporated into the polymer backbone in one emulsion polymerization process. In a 30% PVC semi-gloss paint formulation, latex prepared with the reactive surfactant had about 60% better wet scrub resistance compared to the styrene-acrylic benchmark latex.
In the presentation, “Fluoro-free and silicone-free blocking resistance additives for waterborne coatings,” Susan Dong of Stepan Company described some new phosphate ester additives for formulating coatings with high temperature block resistance.
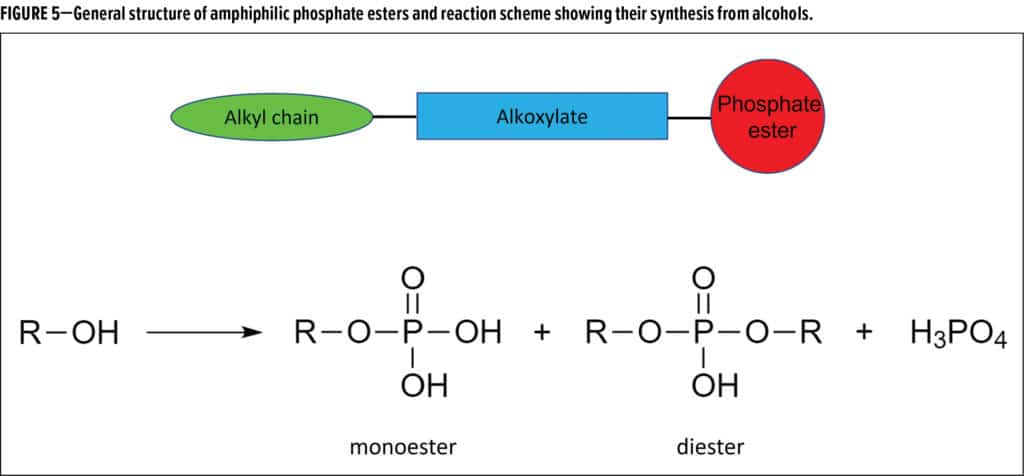 Dong first described some common methods for formulating to better block resistance. One path is to formulate at higher PVC, but that is not always feasible due to its effect on other properties. Silicone-based additives can have issues with incompatibility and lower efficacy, while fluoro-based additives have issues with environmental persistence, incompatibility and cost. The development of two phosphate ester additives was described, with the general amphiphilic structure shown in Figure 5 of a hydrophobic alkyl chain and hydrophilic alkoxylate linking to the phosphate group and formed by the generic phosphatization reaction also shown in Figure 5.
Dong first described some common methods for formulating to better block resistance. One path is to formulate at higher PVC, but that is not always feasible due to its effect on other properties. Silicone-based additives can have issues with incompatibility and lower efficacy, while fluoro-based additives have issues with environmental persistence, incompatibility and cost. The development of two phosphate ester additives was described, with the general amphiphilic structure shown in Figure 5 of a hydrophobic alkyl chain and hydrophilic alkoxylate linking to the phosphate group and formed by the generic phosphatization reaction also shown in Figure 5.
Key variables explored in the study included the length and branching of the alkyl chain, degree of alkoxylation, mono/diester ratio, and type of counterion used to neutralize the phosphoric acid groups.
Block resistance is facilitated by the surface modification and lubrication of the film surface. Chemical structure affects the speed at which the surfactants can migrate to the air/film interface, the concentration at the surface, and the resulting surface energy of the film.
Dong described how dynamic surface tension, contact angle, surface energy measurements, and block resistance testing in paints were used to evaluate the effect of structural variations in the phosphate esters.
Dong described some key findings, including that surface tension increases with degree of ethoxylation, while surface energy of the paint increases and block resistance decreases. Alkyl chains in the middle of the range studied gave faster reduction and overall lower dynamic surface tension, as well as lower surface energy and better block resistance.
Organic counterions such as diethanolamine provided better water solubility for the phosphate ester surfactants, as well as better dynamic surface tension, lower surface energy and better anti-blocking. A range of other amine-based counterions were demonstrated to provide excellent block resistance as well.
Based on the results, Dong summarized that two phosphate ester additives have been introduced which can match the anti-blocking functionality of fluorosurfactants with a better environmental profile.
Reference
- Our Common Future by G.H. Brundtland and the World Commission on Environment and Development, Oxford University Press, 1987.
