By Scott C. Brown, Michael Diebold, Daniel Kraiter, Carlos Velez,and Peter Jernakoff, The Chemours Company
The fouling of exterior coating surfaces by dirt is no simple matter. The process is complex, dynamic, and varies in length and time. It also includes a range of confounding particle transport, attachment, and release mechanisms. While complex, strategic, multiscale testing and analysis combined with particle and surface science fundamentals can be used to isolate the dominant mechanisms. In this article, fundamental concepts in surface and particle science as they relate to dirt pickup resistance are presented. We demonstrate how these concepts were applied to develop a failure-mode-targeted accelerated test method resulting in good correlations with outdoor exposure data. We further examine the impact of microscale and nanoscale surface features on the fouling mechanism through direct surface interaction measurements and imaging on commercial coating surfaces. A path forward towards development of a comprehensive, yet practical understanding of dirt pickup resistance is proposed.
Introduction
Exterior architectural coatings represent a significant percentage of the volume and value of the coating market. The global exterior architectural coatings market size was valued at over $20 billion in 2017 and is anticipated to approach $30 billion by 2025.1,2 With increasing demands for latex exterior paints and more stringent volatile organic content (VOC) restrictions, dirt pickup has become an area of attention for the paint industry. Compared to solventborne paints, latex paints, and particularly those with low VOC content, tend to be more susceptible to dirt pickup on exterior surfaces. Dirt pickup resistance (DPUR) is an important performance attribute for exterior architectural coatings, especially in growing, highly populated markets that have poor air quality. DPUR refers to the ability of a surface to resist discoloration due to the deposition of particles from the environment. Durable exterior coatings of high DPUR maintain their attractiveness for longer periods of time and offer more sustainable coating solutions due to reduced consumption of coating ingredients and related waste generation. Consumer desire remains strong for the ideal exterior paint—one with low dirt pickup and long durability—but delivering it continues to pose a challenge for manufacturers.
Several documented studies over the past three decades provide guidance on how to engineer high DPUR coatings.3-8 As early as 1996, many of the general coating formulation considerations for latex paints were documented.3 These include factors such as pigment volume concentration (PVC), resin/paint type, resin glass transition temperature (Tg), TiO2 pigment, rheology modifiers, fillers, and surface wetting. In 2002, using a large collection of outdoor testing data, Wagner and Buamstark4 ranked formulation constituents based on their relative impact on DPUR performance. Notably, the authors identified formulation PVC and binder selection as the most consistent, strongly influencing variables. The type of formulation and selection of pigment were other potentially strong—yet less consistent (and sometimes negligible)—considerations. However, this knowledge by itself is insufficient to design high-performing DPUR coatings without substantial outdoor testing.
The coating formulation is an essential piece of the puzzle, but the important factor for DPUR is how the coating surface develops after the application and curing process. Ultimately, the surface properties of the resulting paint film define the interaction with environmental particles, weather, and other variables that impact the dirt accumulation process. Environmental properties, such as precipitation and ultraviolet light irradiation may further alter surfaces over time, further impacting DPUR performance.3,4,9-11 Figure 1 provides an overview of how coating formulation parameters and exterior environmental conditions affect coating surface properties that ultimately define dirt pickup.

While it is understood that a number of surface properties can influence DPUR, current laboratory testing procedures for DPUR performance are not designed to address the broad array of considerations required for robust predictability. Indeed, the testing protocols in academia and industry are scattered. Moreover, the methods are diverse and tend to look at only a subset of the mechanisms that may be important for DPUR. As a result, the assessment of DPUR performance is just as dependent on outdoor exposure testing today as it was at the beginning of this century.
The present study represents the first systematic attempt to gain an improved fundamental understanding of DPUR through targeted, mechanism-based experimentation. Using foundational concepts from aerosol and colloidal science, a series of laboratory tests have been developed that evaluate DPUR failure mechanisms. These tests have been used in conjunction with outdoor exposure studies to identify dominant mechanisms impacting the DPUR performance of a series of commercial exterior architectural coating formulations. This approach, in general, can be applied to accelerate formulation development, clarify regional DPUR differences, and perhaps to identify new concepts for improving DPUR performance. The basic concepts explored, associated testing, and their correlations with outdoor exposures are outlined below.
Mechanisms of Dirt Pickup
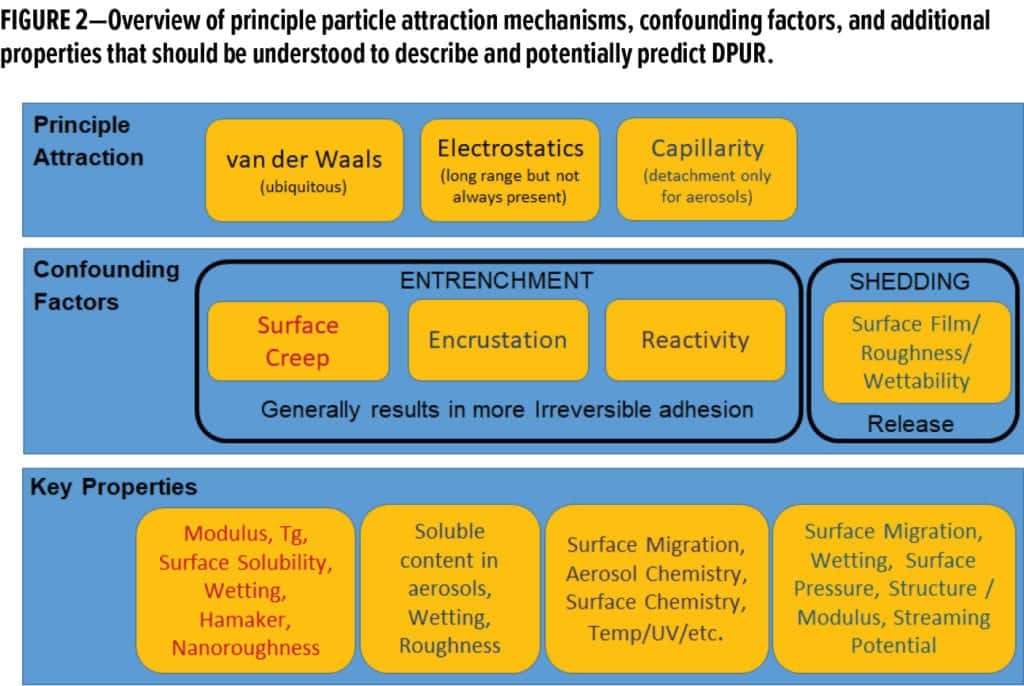
The collective phenomenon of dirt pickup results from a range of mechanisms based on different aspects of particle and surface adhesion science. At a very basic level, DPUR is simply related to the time-dependent accumulation of environmental particles onto surfaces. For simplicity, the complex considerations in surface adhesion (Figure 2) for the process of dirt accumulation on a coating surface were broken down into three primary events: (1) deposition, (2) adhesion and entrenchment, and (3) shedding and release. The basic phenomena likely to contribute to each of these events are briefly presented below.
Deposition
It is well known that the regions around the world with the most air pollution have higher rates of dirt accumulation on surfaces. Soot particles have been identified and confirmed by many to be the primary bad actor in the degradation of the appearance of exterior surfaces in urban areas. In rural areas, the local dirt composition tends to play more of a role, but in general the carbon in soot remains the dominant discoloring agent on light-colored surfaces. In the DPUR community, there is mixed messaging on the dominant factors that control dirt deposition on these coating surfaces. Some publications imply that the deposition occurs from dew, rain droplets, and other liquid transfer events—this is also implied by the types of testing employed (e.g., slurry-based)—while others infer that it occurs predominantly through a dry deposition phenomenon.
From an aerosol science perspective, the deposition of particles on surfaces from aerosols (liquid droplets or dry particles) is well studied. Deposition on vertical surfaces tends to be controlled by static electric effects and van der Waals forces. In some cases, wetting and capillarity can play a role, but even in those situations either van der Waals or static electric effects will operate first, guiding contact and defining initial capture. Unlike capillary forces, van der Waals and static electric forces operate before surface contact (i.e., before the particle physically touches the surface). Notably, van der Waals forces only act over a few tens of nanometers, while static electric effects could act above the millimeter length scales. From a probabilistic perspective, if deposition is controlled by van der Waals forces, the distances are so small that it would be a challenge to see differences between surfaces, whereas stark differences in deposition would be seen if static electric properties played a role.
To better understand whether the deposition step makes a difference in DPUR, controlled testing of particle attachment on paint films is needed. In particular, it is important to determine whether static electric effects are important to consider. It is also important to determine if slurry-mediated deposition or aerosol-based deposition is more consistent with outdoor DPUR performance.
Adhesions and Entrenchment
The factors that control particle adhesion are much more complicated than those that control deposition. Indeed, van der Waals forces, static electric effects, ion electrostatics, capillarity, and many more complications can play a role (Figure 2). A simplifying approach for looking at particle adhesion is to focus on events that are likely to lead to strong or irreversible adhesion, otherwise known as particle entrenchment. The rationale here is that irreversible adhesion events should result in a consistent dirt pickup and discoloration over time with potentially less dependence on exterior exposure conditions. Reversible adhesion events would, however, be much more chaotic and likely more sensitive to local weather-driven events. In other words, if a consistently dominant mechanism for DPUR exists, it should, in theory, result from a strong adhesion or entrenchment event. These are the mechanisms that formulators and ingredient manufacturers would want to target to have the most impact on DPUR.
Three strong adhesion (entrenchment) mechanisms were identified conceptually and probed in this study. They included two different surface creep events and one encrustation event. Surface creep refers to situations where the surface matrix is softened or transformed into a liquid or liquid-like substance, enabling it to engulf particles. Encrustation is where soluble components from the particles or aerosols result in the gluing down of particles onto the surface as they dry. These mechanisms are presented in the Euler diagram in Figure 3.
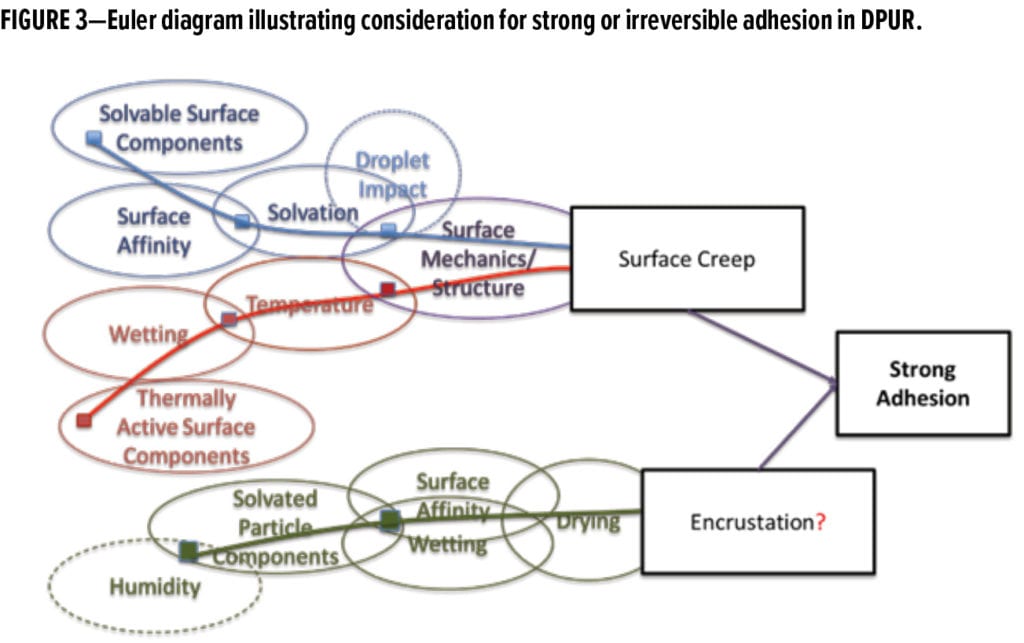
Precipitation- or water-induced creep is based on the possibility that the outermost portion of the surface becomes solvated or mushy when wet, allowing it to engulf or grip onto particles as it dries. This effectively glues particles down, making them more difficult to release. This mechanism would be highly dependent on the water solubility of the outermost coating surface and its properties. A similar phenomenon can happen when the dissolving material is carried with the particles, referred to as encrustation. Under this scenario, particles are transported in droplets containing dissolved materials, or particles consisting of salts or other water soluble (or partially soluble) substances are transported to a surface where they become wet, and, through a drying processes (e.g., rain and dry, cyclical humidity cycles), become affixed to the surface. Both mechanisms previously described rely on the dissolution, or partial dissolution, of materials and their ability to bridge the particle and surface upon drying. The encrustation mechanism requires that the “glue” is carried with the particles or is from the environment, so it is much more dependent on local conditions than the surface creep-induced mechanism. Because encrustation is likely scenario-specific and regionally dependent, it is expected to be a minor (one-off) contributor and is not an explicit focus of this study.
Lastly, thermal surface creep is another potentially important strong adhesion mechanism that could occur (Figure 3). Thermal surface creep occurs when the outermost layer of the surface matrix becomes softened or melted due to an increase in temperature. When this occurs, the surface can adhere to, wet onto, or engulf the particles. This mechanism would be highly dependent on the surface composition and the Tg and other thermal properties of the outermost surface constituents (e.g., resin).
To better understand the importance of strong adhesion mechanisms in DPUR, targeted experiments are needed that directly compare the mechanisms with outdoor DPUR performance.
Particle Shedding and Release
For long-term DPUR performance, particle release, and surface shedding effects can be important factors. Surface transformations and remodeling can occur due to surface heterogeneities, environment-induced physical and chemical transformations (e.g., chalking), dissolution of paint components, or simply due to the accumulation of particles on the surface that enable inertial effects to take over. The blooming of surfactants and other soluble paint components to the surface is also known to lead to localized particle release and is often characterized through leaching resistance studies.
A pertinent question in DPUR is, “What does it take to remove dirt from a surface without human intervention, and is this important for overall DPUR performance?” A number of DPUR tests have employed shaking or tapping of bulk powders to determine if clumps of dirt can be removed from a surface. Is this realistic for real-world DPUR that occurs from suspended particles in the atmosphere?
To simplify the complexity in this area, the physics around both particle deposition and release are briefly reviewed. When dealing with particle systems, there exists a balance between the impact of inertial events (e.g., gravity, momentum from wind, precipitation), and surface forces (e.g., van der Waals adhesion) that almost always needs to be considered regarding whether bulk or surface interactions are dominant. The particle Péclet number12 provides a useful gauge of where a particle (or cluster of particles) lies on this spectrum (Equation 1) as the balance between Stoke’s Sedimentation and Brownian motion:
Pe = 2πDrga4/3kT (1)
where Dr = effective difference in density between the particle and the continuous phase, g = acceleration due to gravity, a = spherical equivalent particle radius, k = Boltzman’s constant, T = Temperature.
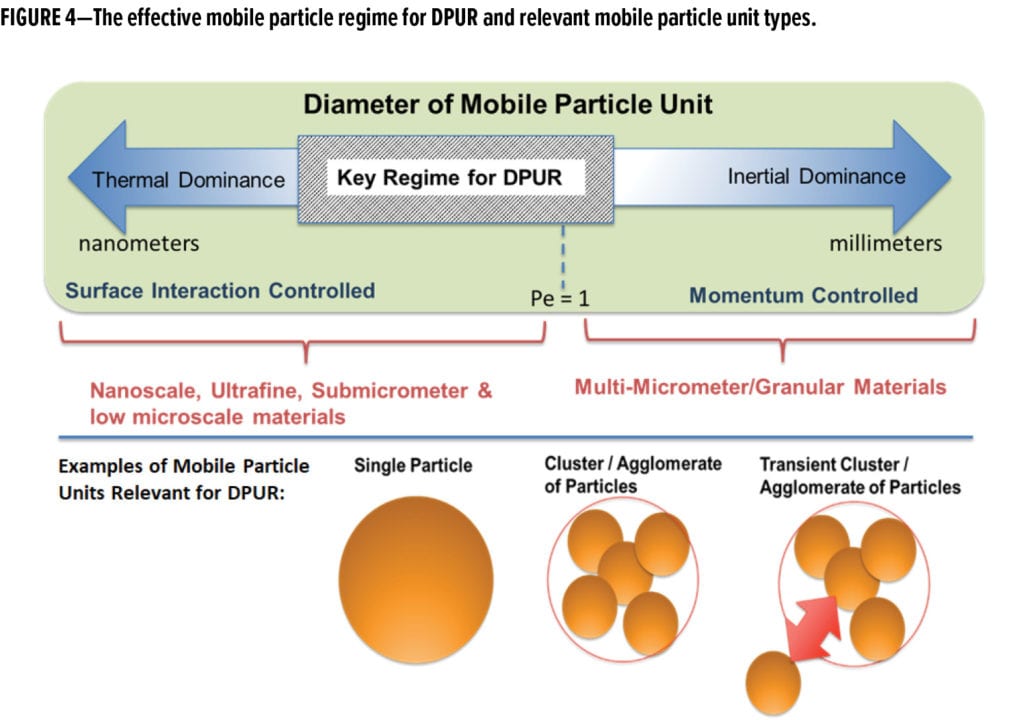
Particles dominated by gravitational interactions (e.g., Pe > 10) are likely to settle rapidly and are not as likely to be available to surfaces. When they do interact, they will be more subject to bulk particle effects. For instance, they are much more likely to settle on flat surfaces and less likely to adhere to a vertical surface as gravity would be pulling them downwards, promoting detachment. On the other hand, particles dominated by thermal interactions (e.g., Pe < 0.1) are fully reliant on surface interactions for deposition. This effect has been seen and noted in a number of studies investigating the influence of pollution on historical structures, wherein it is noted that smaller particles tend to foul vertical surfaces such as exterior walls.11 The mass median diameter of typical outdoor aerosols in urban environments is approximately 2 to 4 mm in size,14 with a corresponding number median of about 100 nm. These values vary with distance from the emission source,12-14 and such particles have a Pe well below 0.1.
In aerosol science, particles in the range of 50 nm to 2 mm are in the accumulation size range13 (i.e., actively accumulate in the atmosphere), allowing the particle to travel and deposit on structures far from the emission source. Particles smaller than roughly 50 nm are more susceptible to coagulation and less stable as aerosols (i.e., grow to larger effective mobile particle size), and larger particles rapidly settle. Non-settling airborne particles must have a low Pe and, therefore, exist as small particles (aerodynamic diameter) and clusters of low inertia. Bulk powders, on the other hand, are predominantly agglomerated as large particles that settle quickly, and the vast majority of the clusters are governed by inertia with a Pe on the order of 100 and approximate sizes above 20 mm. Even if the bulk powder is composed of fine or ultrafine particles, their behavior will be dominated by the agglomerate structure.
This phenomenon immediately suggests that testing DPUR using bulk powders may represent a different physics than the actual environmental process of dirt pickup due to the Péclet regime. The use of bulk powders, or larger-sized particles, may primarily look at differences in strong forces such as capillarity and electrostatics, whereas the universality of van der Waals may be dominant under more typical aerosol interactions. Indeed, for a number of studies exploring fouling on important architectural structures, it is indicated that although capillarity can enhance particle deposition efficiency, the predominant deposition process appears to be from the dry state and likely from van der Waals interactions simply because it is operating for most, if not all, of time.11
The accumulation regime is the key regime for consideration in DPUR (Figure 4) by Pe. This explicitly suggests a careful look at the surface forces and surface properties because these mechanisms are dominant and should control the prevailing deposition and release processes. To impact the appearance of a coating surface, interacting dirt particles (i.e., mobile particle units, see Figure 4) must have small effective sizes such that they are not immediately released from a surface due to gravity (wind or precipitation).
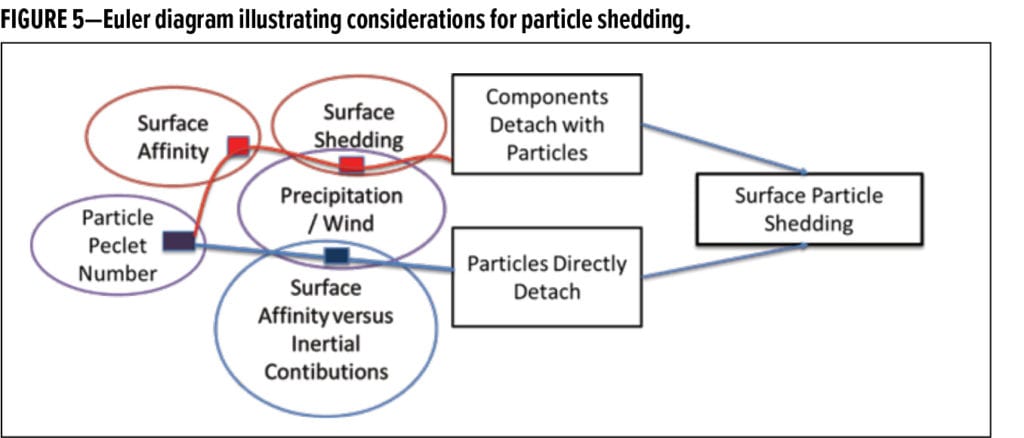
Understanding that the particles in dirt pickup processes are originally in the low-Pe regime is important. This consideration is fundamental to ensuring that appropriate release scenarios are investigated. For the particles to be released, this explicitly states that it must either be: (1) a surface interaction mechanism, (2) involve the growth of the effective mobile particle unit to larger sizes, or (3) both of these mechanisms. A simplifying consideration is that as smaller particles build up on a surface, they may agglomerate to large sizes that can shed (e.g., with the assistance of rain or wind) by inertia. Two basic mechanisms for particle shedding are (1) the case where particles attach to a surface that itself dislodges due to physical and chemical means (e.g., chalking or degradation of the underlying surface), or (2) the case where the particles form large clusters on the surface that enable inertial release. These mechanisms are shown in the Euler diagram presented in Figure 5.
While particle shedding is clearly possible, it is unclear to what degree particle shedding is important to DPUR in modern formulations and under the desired product lifetimes. To better understand the relative contribution of particle shedding, experiments are needed to determine the degree to which wind or precipitation may shed particles from a discolored paint film and, if important, which underlying factors control these phenomena.
Through this simplified and compartmentalized view of the dirt pickup phenomenon, laboratory tests were designed to identify which mechanisms and considerations were important in deposition, adhesion, and release as determined by comparisons to the outdoor performance of a series of commercial paints exposed at test facilities in Chennai, India, and Guangzhou, China.
Experimental
Materials
Dirt Simulants
To simulate soot, Degussa Flamruss 101 carbon black was used. In select experiments, iron (III) oxide, red (Hematite) (99.8%-Fe) was used as a colored, cationic, hydrophilic dirt simulant.
Paint Panels
Control and experimental paints were drawn down by hand on 30.48 cm long x 10.16 cm wide x 0.06 cm thick Q-Lab aluminum panels using a 0.10 mm gap clearance, stainless steel BYK-Gardner bar film applicator in conjunction with a Paul M. Gardner Co. stainless steel vacuum plate. The resulting wet paint films were dried indoors for seven days under ambient laboratory lighting conditions at a temperature of about 20 °C and a relative humidity of about 50%. Paint film dimensions after drying were as follows: 27.94 cm long x 7.62 cm wide x between 0.06 mm and 0.11 mm thick. Paint film thicknesses were determined using a Dualscope FMP40C measuring device.
Commercial Paints
Seven commercial exterior architectural paints were used in this study. Each of the paints investigated claimed dirt pickup resistance properties. Three semi-gloss, two sheen, and two flat paints were evaluated. Although the formulations of these paints were unknown, it is important to note that two pairs of the paints were from the same commercial line but were different types, or sheen levels. Details of the paints primarily used in this study are provided in Table 1. Note that Paints 8 and 9 were used only in the slurry treatment experiments.
Methods
Outdoor Exposure Testing
Paint panels were sent for outdoor exposure to testing sites in Guangzhou, China and Chennai, India. Outdoor exposure testing was initiated per ASTM test methods G147-2009 and G7-2013 and in accordance with the generally recognized governing standards for the outdoor evaluation of paint. The panels were mounted facing south at a 45-degree angle from horizontal on an aluminum exposure rack. Starting at the zero hour exposure time point, spectral measurements of the paint films were performed at periodic intervals per ASTM test methods E1349-06 (2013) and E308-2013 using an X-Rite 948 reflection spectrocolorimeter (D65 CIE standard illuminant, 0-degree illumination angle, 45-degree viewing angle), or via ASTM test method E1331 using an X-Rite Color i7 spectrophotometer (D65 CIE standard illuminant, 0-degree illumination angle, 10-degree viewing angle, specular reflectance excluded). Each measurement consisted of gathering spectral reflectance data from three widely separated areas of a paint film and averaging the results to produce a corresponding HunterLab color scale-based average L* value (light-dark color axis). Obtained average L* values were used to calculate an average dirt pickup value (average DL*) for each paint film at various exposure time points using the equation:
Average DL* =
(0 hour exposure, average L*) (2)
– (X hour exposure, average L*).
Note that larger average DL* values equate to greater dirt pickup.
Aerosolized Carbon Black Deposition
The paint panels were cut into chips, roughly 2 x 5 cm or smaller, using a hand-operated Di-Acro metal sheet cutter. The chips were affixed to the inside of a custom aerosol generation chamber capable of dispersing carbon black into a dust cloud in the deposition region that consists of greater than 90% particulate matter (PM) 2.5 content as determined by an SKC PM2.5 IMPACT Sampler. This chamber was developed, in part, to enable an even deposition of particles on paint samples.15 A typical dusting used in this experimentation targets grayscale values in the range of 150 to 200. Prior to dusting, a portion of the paint chip is covered by Scotch 3M Magic tape to preserve the original paint coloration. After dusting this tape is removed.
Grayscale Evaluation
Grayscale evaluation was determined using an Epson Perfection V750 PRO document scanner and accompanying scanner software. Scans were conducted using 24-bit color at a resolution of 400 dots per inch. The average grayscale values (0 to 255, where 0 = pure black and 255 = pure white) were determined using National Institutes of Health ImageJ image analysis software to average multiple regions of interest containing greater than 100 pixels. Grayscale measurements using this method were determined to linearly correlate with L* measurements as determined in the outdoor experiments.
Dirt Simulant Deposition Evaluation
The homogeneity of dust deposition on the paint chips was determined with the use of a document scanner as described above. This process provided both images and grayscale distribution information from the analysis of multiple regions of interest (i.e., selected representative areas within which the analysis occurs). DGrayscale values were determined by subtracting the average grayscale value of the dusted area from the average grayscale value of the original paint (taken as the grayscale value measured from the paint chip area protected by tape during deposition). Larger DGrayscale values equate to more dirt deposition.
Evaluation of Precipitation/Water-Induced Surface Creep
Pre-dusted Surfaces: 1 x 4 cm paint chips were dusted to approximately equivalent grayscale values using Flamruss 101 carbon black. Subsequently, the paint chips were incubated in either pH 3 water or deionized (DI) water for 30 min at room temperature. The paint chips were incubated in a manner where only a portion of the chips were submerged in liquid. The paint chips were removed and allowed to air dry for 24 h Afterwards, Scotch 3M Magic tape was used to remove carbon black that was not strongly adhered to the surface of the section in contact with liquid and the section that remained dry. This process, called “tape peeling” is an effective and reproducible means of removing particles that are only physically attached. The relative graying of the substrate from liquid immersion was determined by subtracting the average grayscale value of the immersed area after tape peeling from the average grayscale value of the dry area after tape peeling.
Slurry Treatment: Slurries consisting of 25 wt% carbon black (Flamrus 101) in DI water and, separately, 25 wt% iron (III) oxide in DI water were prepared and applied to paint film-coated panels via a paint brush, allowed to dry, and subsequently removed as an alternate approach for dirt pickup resistance evaluation by surface creep. For carbon black and, separately for iron (III) oxide, 10 g of powder was added to 30 g DI water and sonicated for four min at 50% amplitude in a Qsonica Q700 ultrasonic processor equipped with a 0.50 in. replaceable-tip horn.
The resulting dispersion was cooled to room temperature and then applied by brush to cover 1/3 of a paint chip to create the slurry treated area. The slurry treated panels were dried for four hours under laboratory conditions, rinsed with tap water and lightly wiped with a wet sponge. This process was conducted in a manner to prevent contamination and discoloring of the untreated area of the paint film. The rinsed and wiped panels were further air dried for another 24 h before scanning. The average grayscale values determined for the brushed area and the control area (original paint) were used to calculate the DGrayscale value for each chip as follows: DGrayscale = (average grayscale value for untreated original paint control area) – (average grayscale value for the rinsed and wiped slurry treated area). Larger average DGrayscale values equate to greater carbon black or iron (III) oxide pickup by a paint film surface.
Evaluation of Thermal Surface Creep
Test paint chips were dusted following the aerosolized carbon black method and subsequently incubated in an oven for three days at 45 °C. After cooling to room temperature, a portion of the carbon black dusted area was tape peeled to remove any loosely adhered carbon black. DGrayscale values were then determined as follows: DGrayscale = (average grayscale value of non-dusted, original paint) – (average grayscale value from the dusted, heat-treated and tape peeled region). Increasing DGrayscale values correlate to increasing heat-induced surface creep.
Atomic Force Microscopy
An Oxford Asylum Instruments Origin+ AFM equipped with a polymer heating stage was used for all imaging. Briefly, 1 x 1 cm samples were cut and mounted to the polymer heating stage. An Olympus AC240 silicon cantilever or a NanoProbes diamond-like carbon spherical probe 100 nm in diameter was used to image the surface in amplitude controlled (AC) mode. Force maps were taken under low amplitude oscillations to enable both AC and normal force curve information. Data was taken at 60 °C unless otherwise noted. Electrostatic force microscopy was conducted at room temperature on an isolated glass slide, using a platinum-coated Olympus AC 240 tip. Briefly, a bias of +5V was applied to the tip engaged and rastered in contact mode on the paint surface to create a 500 x 500 nm-charged area. The tip was disengaged and a one square micrometer image (including the previously rastered area) was imaged in non-contact NAP mode with a +5 V bias to determine if the charge remained.
Particle Shedding Assessment of Films Deposited by Aerosol
Scanned images of carbon black coated paint chips immersed in liquids or exposed to outdoor weather (after heavy dusting) were used to gauge the relative importance and complexity of particle shedding events. Delta grayscale values were determined in a similar manner to the dirt simulant deposition evaluation.
Results and Discussion
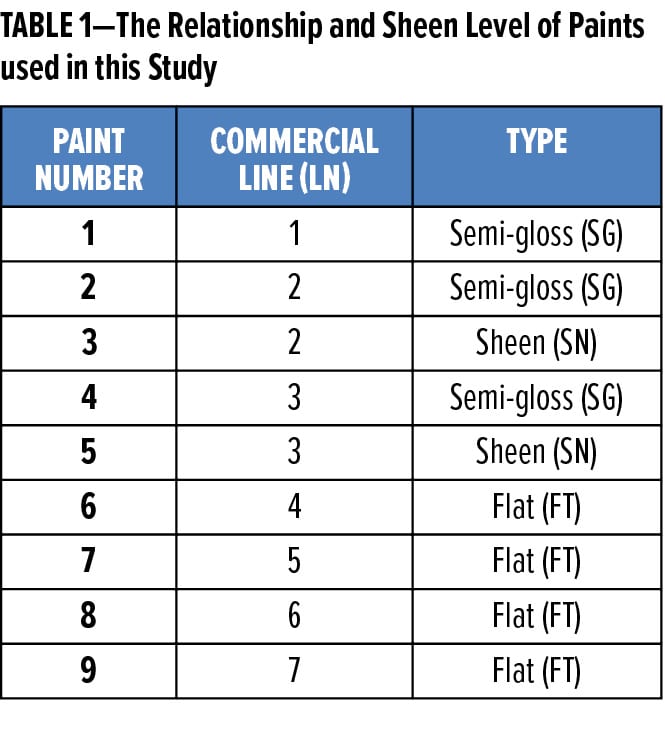
The first step in attempting to deconvolute DPUR mechanisms is to identify a series of paints that demonstrate a range of DPUR performance in outdoor testing. Given the importance of growing Asian markets, outdoor testing sites at facilities in Chennai, India, and Guangzhou, China, were chosen. These two regions are heavily polluted, resulting in relatively quick dirt pickup in exterior exposure testing. Figure 6 provides the DL* results from exposures at these sites.
Significant differentiation between the commercial exterior architectural paints is noted from the early to the later exposure time points. It is noted that the Guangzhou, China site has a slightly more erratic dirt pickup profile than the Chennai, India site. However, the best- and worst-performing DPUR paints agree between the two sites. At early exposure times, the paints appear to fall into three groupings that agree between the sites. Because of the improved distinction among paints at the higher exposure times (e.g., greater than 200 days), these later timepoints are believed to be more sensitive to subtle paint performance differences.
To understand whether the initial dirt deposition event is a defining factor in DPUR, a custom aerosol generation chamber was built capable of dispersing carbon black into an aerosol cloud of greater than 90% PM 2.5 content. It is important to note that the predominance of soot (simulated by carbon black) at both the Chennai and Guangzhou exposure sites was confirmed by SEM imaging combined with energy dispersive spectroscopy and optical microscopy from retained paint panels exposed in the respective areas.
The paints tested in the outdoor exposure experiments were exposed to carbon black—consisting mainly of PM 2.5 content—in the aerosol chamber. The relative amount of carbon black deposited on the samples was estimated by DGrayscale analysis. Increasing DGrayscale relates to increasing carbon black deposition. The average results of three randomized aerosol deposition experiments is provided in Figure 7. An optical image of the result from a dusting experiment performed on many of the paints used in this study is provided in Figure 8.

It is noted that there is little differentiation among the samples within the random variation of the experiment. Further, there is no observable correlation between the visual interpretation of deposited carbon black and the results from outdoor exposures provided in Figure 6. Electrostatic force microscopy experiments were also conducted on several of the paints and no signs of a residual surface charge was observed. This suggests that contributions from static electric-mediated deposition effects for the paints in this study are likely minimal, and indicates that the dominant mode of deposition is through van der Waals forces. This is further confirmed by the even deposition of carbon black on the surface of the paint films as depicted in Figure 8. In the case of PTFE or PE substrates (which do exhibit a static surface charge), exposure to carbon black from the chamber results in carbon black deposition patterns that are not even and appear to highlight electric field lines on the surface.
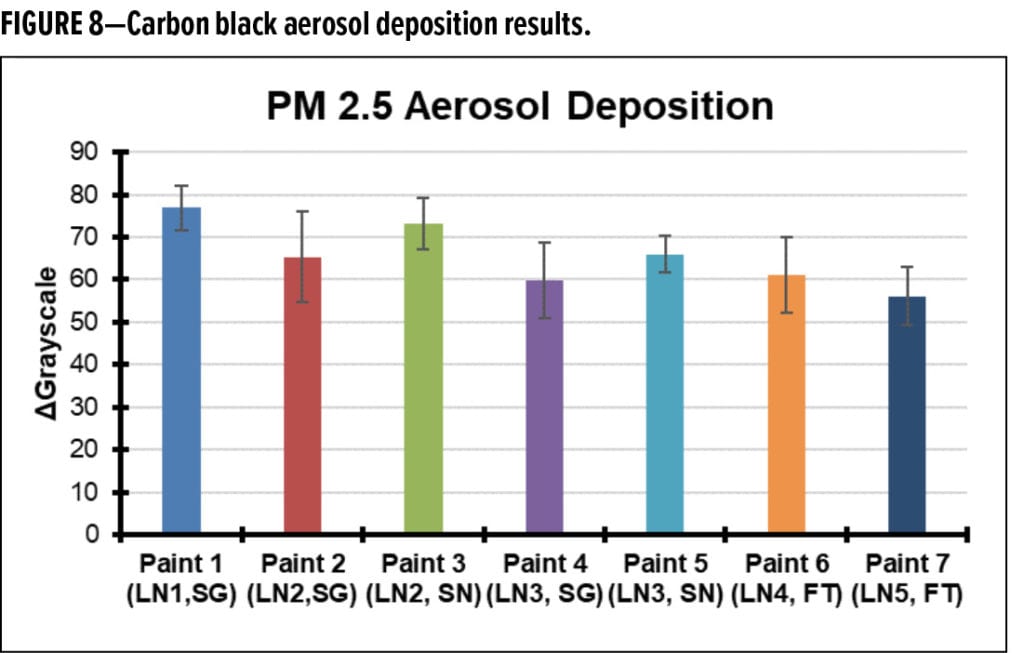
With these observations, it is concluded that dirt deposition does not appear to be substantially differentiated between the test paints in this study. Accordingly, investigations moved towards the analysis of potentially strong—and likely irreversible—adhesion mechanisms. This began with experiments meant to mimic precipitation or water-induced surface creep. Immersion experiments starting with freshly dusted (carbon black aerosol deposition) paint samples were performed using pH 3 water (to simulate acid rain) or DI water (to simulate general precipitation). After immersion in liquid, the paint samples were allowed to dry, then loosely adhered dirt was removed via tape peels. The carbon black remaining on the surface was quantified by calculating the difference in average grayscale value after tape-peel for an area of a paint sample that was dusted with carbon black and not treated with liquid, and area of a paint sample that was dusted with carbon black and treated with the test liquid. This experiment is meant to simulate what might happen if an already dirty panel is exposed to precipitation. DGrayscale results are provided in Figure 9 for the paints used in this study.
The results indicate that flat paints in the present study appear to be more susceptible to this form of strong dirt adhesion, and notably, more so under acidic conditions. While the absolute magnitude of this phenomenon appears to be small, an impact does appear to exist. However, it is important to note that the apparent DPUR performance ranking is not consistent with the outdoor results.
A common DPUR assessment method (that may test the same strong adhesion mechanism) involves creating an aqueous slurry of carbon black, iron oxide, or some other pigment, applying the slurry to test panels, allowing it to dry, then washing off excess materials with some light abrasion (e.g., with a sponge or cheese cloth). After this process is complete, the change in coloration is recorded and used to determine DPUR performance. Because of the prevalence of this procedure in commercial technical product literature,16-18 it was also used in the present study. The results for five of the seven paints present in the exterior exposure experiments are reported in Figure 10.
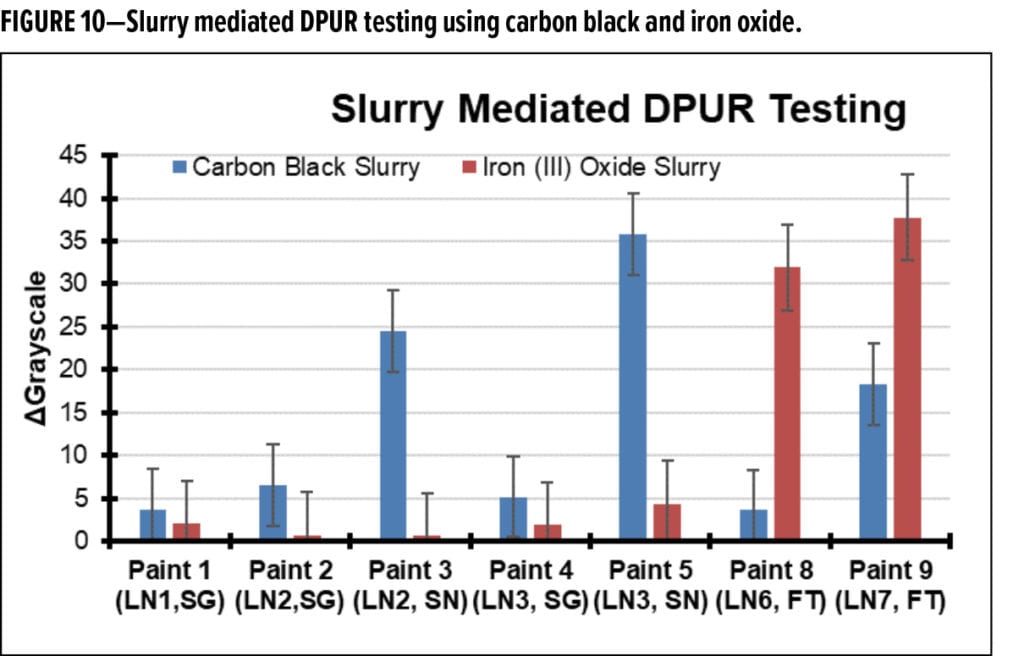
Although, it was speculated that this testing approach might result in an outcome similar to the previously performed water-induced surface creep results (Figure 9), the results do not match. It is not clear if this deviation is due to the prevalence of colloidal phenomena (rather than aerosol interactions) during deposition and early adhesion for the slurry method, or if it is due to some other phenomena. Notably, the particle removal step in the slurry method is less controlled and may result in some removal of the outermost layer of some paint surfaces through abrasion. Also, the underlying paint surface wettability noticeably impacted particle deposition when using the slurry method, whereas dry aerosol deposition was similar among the different paint surfaces.
Differences are seen between the testing performed with carbon black slurries and that performed with the iron (III) oxide slurries. Carbon black slurries seem to highlight the sheen paints as being the most prone to dirt pickup; however, in the outdoor experiments, these were consistently the best performers. Iron (III) oxide slurry testing also did not correlate with outdoor experiments, but correspond with the water-induced surface creep results (Figure 10). These results further indicate that the two flat paints may be more prone to liquid-mediated particle adhesion or entrainment—and given the iron (III) oxide results—a possible greater susceptibility to rust stains. From a physical process point of view, these slurry-mediated tests are more related to indirect dirt pickup from run-off events (e.g., rainfall-induced movement of dirt collected on window sills, roofs, to vertical surfaces) than the events related to the direct deposition from airborne particle matter.
Collectively, these results suggest that water-based particle entrenchment mechanisms are likely not dominant in the outdoor exposure experiments. The worst-performing paint from the outdoor exposure series (Figure 6), performs well in the laboratory surface creep (Figure 9) and slurry-mediated DPUR tests (Figure 10). Indeed, the results in these laboratory tests are generally opposite of what is observed outdoors. That is, the worst performers in the laboratory tend to be the best performers outdoors.
These experiments led to the conclusion that liquid-mediated adhesion events are not strongly influencing outdoor dirt pickup in the present study. Considering that many organizations have been applying slurry-based testing for determining DPUR performance—for decades—this result was surprising.
Next, the potential impact of thermal surface creep was tested. Paint samples were dusted with carbon black and then incubated in an oven for three days at 45 ˚C. This temperature was chosen as a typical elevated surface temperature that is observed on both white and black panels in outdoor experimentation. After thermal incubation, the paint samples were allowed to cool to room temperature, and loosely adhered carbon black was subsequently removed by tape peels. It should be noted that at room temperature, tape peels yielded grayscale values identical to those of samples not exposed to carbon black (i.e., all of the applied carbon black was effectively removed from the surface by tape peels when temperature or water is not applied).
The DGrayscale results from the thermal surface creep experiments are provided in Figure 11 and plotted with the outdoor DL* results from 363 days of exposure in Guangzhou and, separately, in Chennai. Notably, for the first time, the directional trend from outdoor exposure results, as compared to the laboratory experiment, are similar. Linear correlations between the dirt pickup experiments performed outdoors and the laboratory thermal surface creep method were subsequently determined. Two example regressions are given in Figure 12, one for each region. A plot of the linear correlation coefficient (R2 values) vs time for the regression between the grayscale values for laboratory thermal surface creep method and different timepoints in the outdoor dirt pickup experiments are provided in Figure 13.
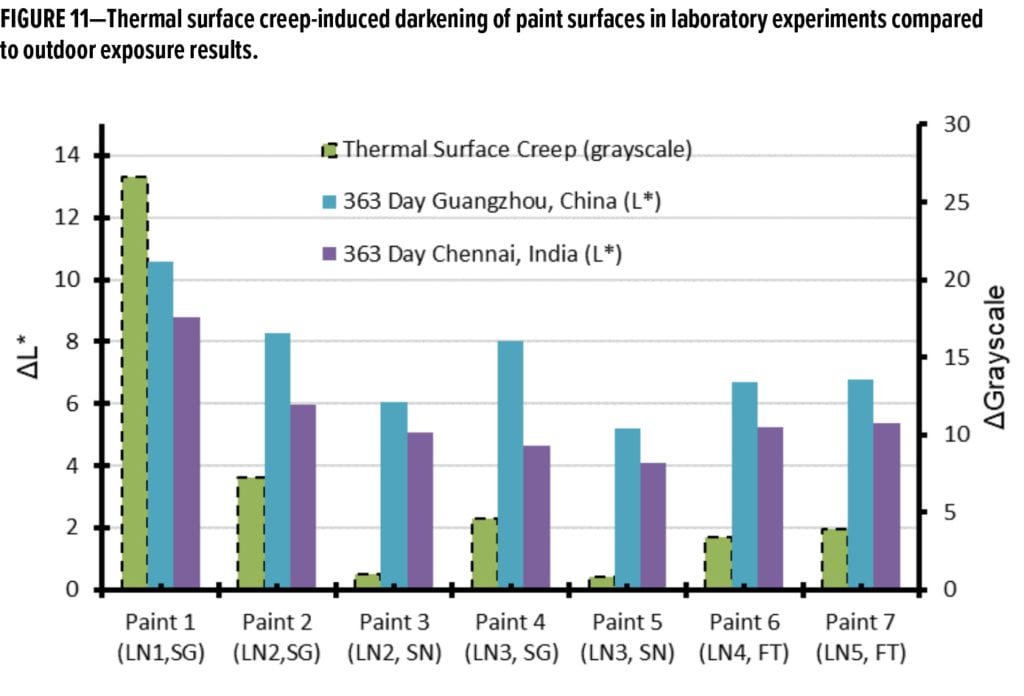
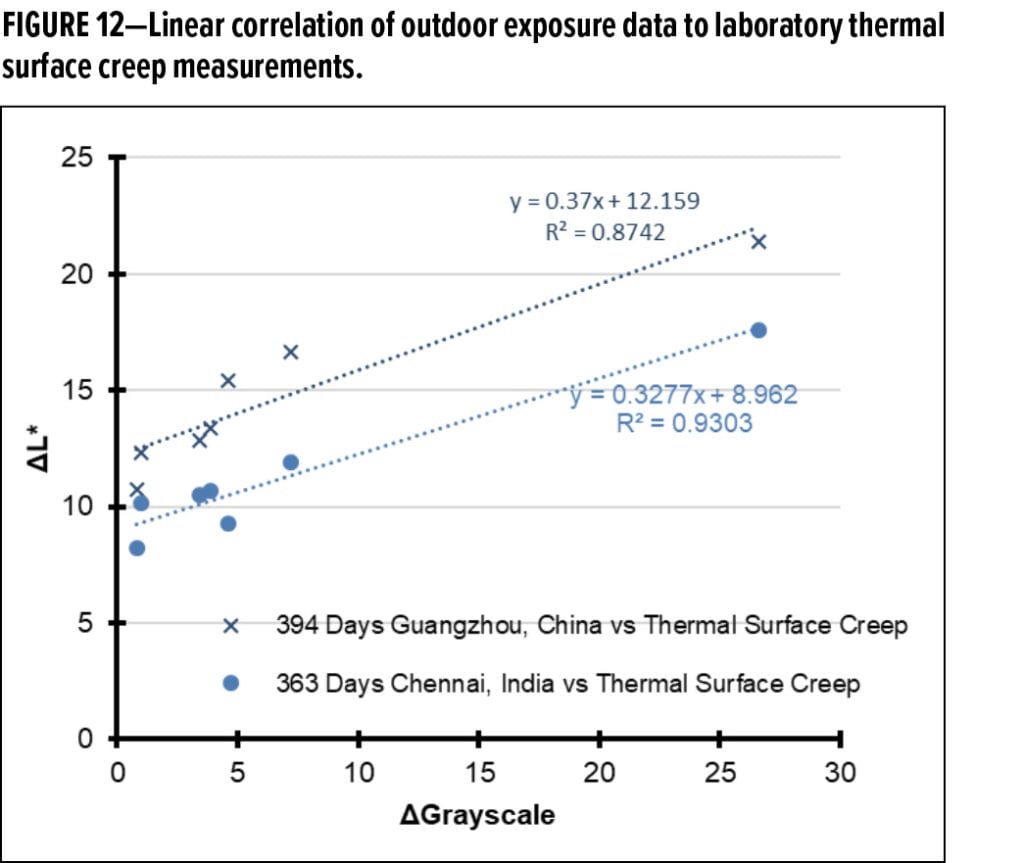
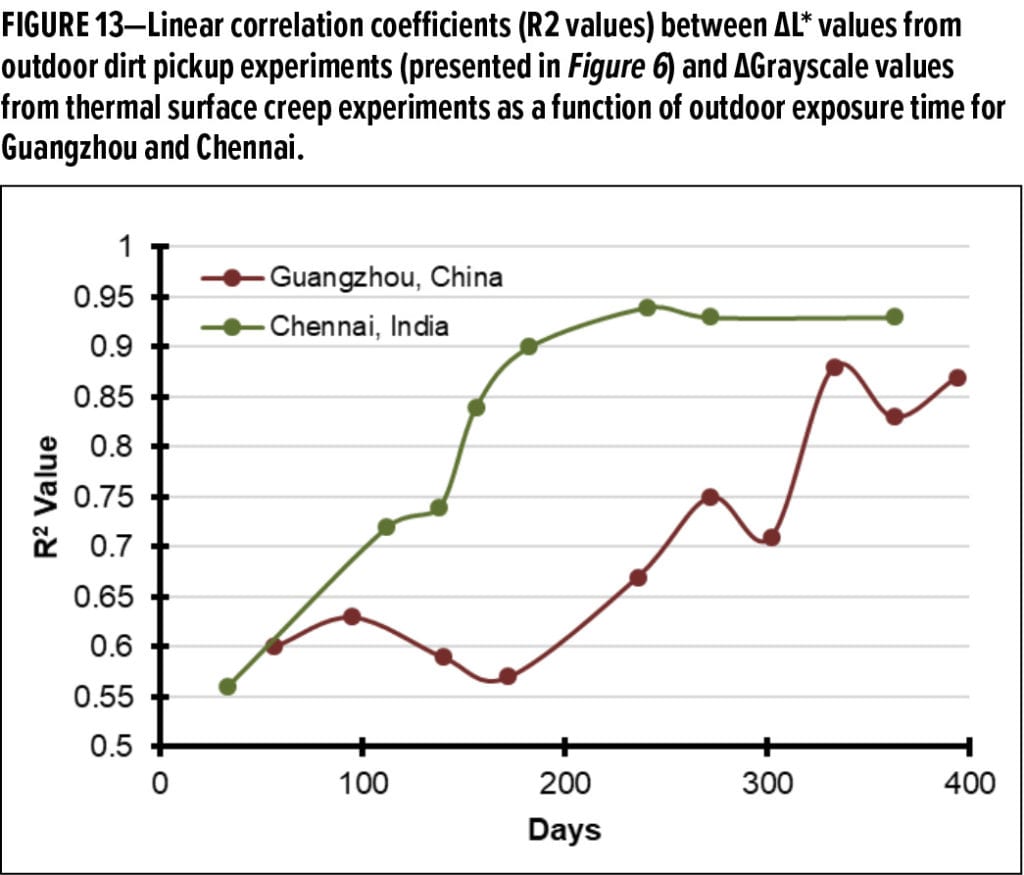
It is noted that the linear correlations between the laboratory experiment and the outdoor dirt pickup results improve with time. At both sites, at exposure timepoints approaching or exceeding one year of exposure, the R2 values approach or exceed 0.9. This is significantly better than any previously reported correlation between outdoor DPUR experiments and DPUR laboratory tests known to the authors.19 This correlation improvement also occurs as the resolution of the outdoor experiments increases (due to further differentiation of the experimental paints in the outdoor dirt pickup experiments in Figure 6). These observations lead to the conclusion that temperature-induced surface creep is the dominant strong adhesion (entrenchment) mechanism for the paints in this study.
To further understand the origin of this phenomena, atomic force microscopy phase contrast images of the surfaces of the paint films were obtained at elevated temperatures and overlaid on the surface topography profile from the corresponding surface height image. These micrographs (Figure 14) provide an indication of “adhesive” and “repulsive” areas on the paint film in combination with the topography. Adhesive, or softer, areas are indicated by the light purple regions, and hard, repulsive surfaces are indicated by the brown regions. The images in Figure 14 were taken at identical settings and sample preparations with the same tip.
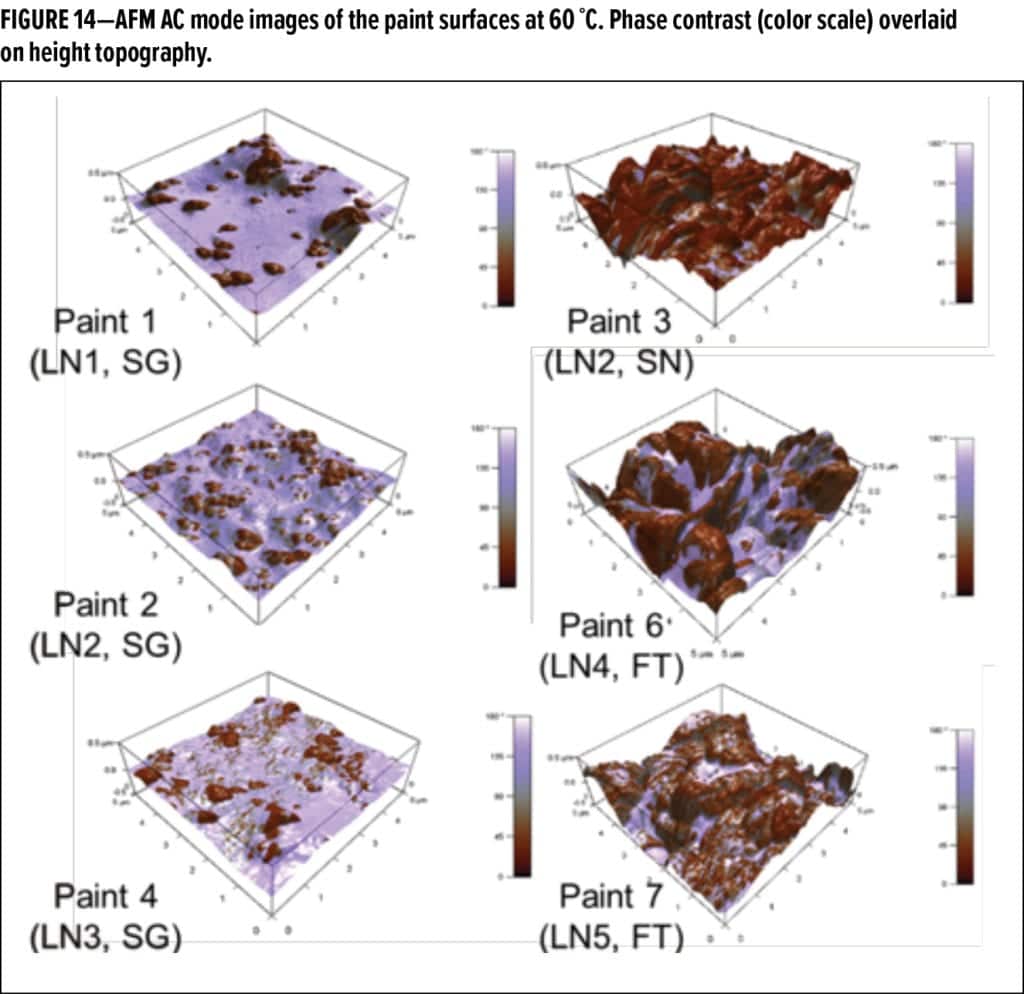
Note that there is a clear difference in coloration between the paint samples presented on the left portion of Figure 14 and those on the right. The paint surfaces on the left clearly have more “soft and sticky” areas at temperature, and those on the right tend to be rougher, with less “soft and sticky” areas. The proportional amount of light purple regions appears to correlate well with both the outdoor DPUR experiments (Figure 6) and the thermal surface creep testing (Figure 11), thus further supporting the proposed mechanism.
Quantitative interaction force measurements were also performed on the surface of the paint films near room temperature, at 45 °C and at 60 °C. Briefly, a 100 nm spherical carbon tip was brought into contact with the paint surface and allowed to press onto the surface until a load of 15 nN was reached. Afterwards, the carbon particle (attached to the AFM tip) was pulled off the surface. The distance that the particle traveled into the surface prior to reaching the 15 nN load was recorded along with the work of adhesion preventing detachment. This procedure was applied to create force maps over several micrometers on the paint surface, thus fingerprinting the surface creep (distance traveled to reach 15 nN after surface contact) and the respective work of adhesion distributions for each paint sample. It is noted that 15 nN represents the approximate self-loading force of a typically sized soot particle through van der Waals interactions. In other words, when a soot particle deposits on a surface it continues to exert a van der Waals force on that surface on the order of 15 nN. Hence, the experiments herein are intended to reflect the degree to which deposited soot particles would sink into the surface (via surface creep) and also the relative difficulty for removing that particle from the surface via the more classical work of adhesion assessment under these self-loading conditions.
At room temperature, there was little to no differentiation among the paint surfaces; however, at higher temperatures, clear distinctions became evident. These results also correlate well with the outdoor exposure results, the laboratory thermal surface creep method, and empirically follow the phase contrast imaging results provided in Figure 14. Example force maps representing the work of adhesion for a Good DPUR paint and a Poor DPUR paint are provided in Figure 15 at different temperatures as an illustration of the effect. Work of adhesion in these images increase as the color changes from black to brown to purple to white. For the paint surface with poor outdoor DPUR performance, there is a clear onset of adhesion with temperature. This is not seen for the paint with good outdoor DPUR performance.

Collectively, this assessment has, thus far, individually tested the importance of the deposition and strong adhesion events as they relate to outdoor DPUR performance. The results clearly indicate the importance (and, in this study, a clear dominance) of thermal surface creep in the DPUR performance of paint films. Not only was a simple test method developed for evaluating thermal surface creep through simple laboratory experimentation using aerosolized carbon black, a common laboratory oven, some tape, and a document scanner, but the fundamental mechanisms were also demonstrated and evaluated at the nanoscale and single particle level using advanced surface force spectroscopy methods.
While a good correlation with a simplified laboratory test and outdoor DPUR testing was found, the question relating to the potential impact of the release of particles remains. Several experiments were performed to determine the relative significance of these mechanisms. While the clear dominance of the thermal surface creep mechanism discussed above suggests that these events may be of little impact (at least in the present research), they may be important in other situations—especially when chalking or heavy particle burdens may be present.
To explore a situation involving a large amount of dust accumulation, several test chips were excessively dusted with carbon black and exposed to the elements outdoors in Wilmington, Del. Results from seven days of exposure are pictorially presented in Figure 16. A substantial influence of the underlying paint surface properties on particle shedding was observed. The paint formulated above Critical Pigment Volume Concentration (CPVC) showed improved shedding over a commercial paint identical to Paint 1 (LN1, SG) shown to the far left in Figure 16. While improved shedding was observed for the above CPVC paint, a much broader distribution of grayscale values is also noted that detracts from its appearance. On the right portion of the image, the same paint is provided at two different levels of surface hydrophobicity. It is noted that the more hydrophobic paint retains more carbon black and the surface remains more evenly shaded (better appearance); whereas, the more hydrophilic version sheds more of the carbon particles but suffers from water spots from a precipitation event.

While it is not expected that these mechanisms become dominant until extremely long external DPUR exposures—or for surfaces that are more horizontal than vertical—this example draws attention to the issue of surface appearance in DPUR. While most testing involves averaging the discoloration on substrates irrespective of how the discolorant is distributed on the surface, one wonders if better characterization of surface appearance, along with averaged surface discoloration in external DPUR exposures could lead to new opportunities.
Conclusions
A systematic and mechanistic failure-mode evaluation of the DPUR performance of commercial coatings has been described. The process was applied to reduce the very complex and chaotic phenomenon of dirt pickup on exterior surfaces into a series of simplified scenarios that could be tested in a laboratory. Corresponding test methods were developed, and through correlation with results from outdoor DPUR testing (at multiple sites), the dominant mechanism impacting the DPUR of seven commercial paints of unknown formulation was determined. The mechanism was also confirmed by direct particle interaction measurements.
Although initial particle deposition events are often speculated as being important for defining the DPUR performance of paint films, the present study indicates that the effect is minor. Rather strong adhesion events, or particle entrenchment, was found to be the dominant process offering differentiation between commercial coatings. Specifically, thermal surface creep was identified as a dominant mechanism in this study and appears to be a major contributor for the DPUR performance of commercial exterior architectural coatings, in general. Many existing DPUR tests often employed by major manufacturers are not sensitive to this mechanism. As of now, much of industry reports results from slurry-mediated testing to evaluate DPUR. While these (slurry-based) strategies may be of significance in some cases, they were found not to be relevant and rather misleading in the present study. These results stress that the coating community should look at DPUR from the perspective of multiple failure mode mechanisms, and much more attention needs to be paid to the thermal surface creep mechanism.
Throughout the process of DPUR mechanism evaluation, a simple laboratory test was developed and applied providing highly correlated (and predictive) results compared to the outdoor DPUR performance at two different exposure sites. To our knowledge, this is the first reporting of a laboratory DPUR test capable of achieving correlation coefficients greater than 0.8 with real-world exposures.
An example of how to undertake failure mode evaluations for DPUR is provided through example. Further extension of this strategy to confirm underlying mechanism through microscopic and nanoscale evaluation of the paint surfaces at relevant environmental conditions is also provided. Additional considerations related to surface appearance and particle shedding are introduced for further discussion and exploration.
Collectively, the results from this study are supportive of the early findings of Wagner and colleagues. In particular, the impact of resin, resin Tg, and the various formulation parameters that define the composition of the outermost surface (and its susceptibility to temperature) are confirmed. The impact of formulation parameters, though not discussed in detail in the present study, have been further explored using the testing strategies discussed herein and are the subject of a future publication. Correlations from the outdoor testing of a broader series of commercial paints, along with custom formulations are ongoing, and the thermal mediated surface creep mechanism appears to be a major contributor in most cases.
Acknowledgements
The authors gratefully acknowledge the assistance of laboratory technical personnel for their dedication and skill analyzing samples used in this study. The people working with the authors in this regard included Mike Milone, Hing Yim, Maria Hassel, Ken Tarburton, Chris Greever, Len Redkoles, Tom Kaczmarczyk, and numerous other individuals.
References
- Pianoforte, K., “Exterior Architectural Coatings Market,” Coatings World (Nov. 2019); accessed January 2020 at: https://www.coatingsworld.com/issues/2019-11-01/view_features/exterior-architectural-coatings-market-
967701/. - Androit Market Research. Global Decorative Paints and Coatings Market Size 2017 by Product (Primer, Enamel, Emulsions and Others), by Technology (Waterborne Coatings, and Solventborne Coatings), By End Use (Residential and Commercial) by Region and Forecast 2018 to 2025. (Oct. 2019); accessed January 2020 at: https://www.adroitmarketresearch.com/industry-
reports/decorative-paints-and-coatings-market. - Wagner O. and Smith, A., “Factors Affecting Dirt Pickup in Latex Coatings,” J. Coatings Technology, 68 (862), 37 (1996).
- Wagner O. and Baumstark R., “How to Control Dirt Pick-Up of Exterior Coatings,” Macromol. Symp., (187), 447-458 (2002).
- Vandezande G., “Improved Dirt Pickup Resistance Critical to Future Coatings Innovation,” Paint & Coatings Industry, 24, 96-100 (2008); Accessed January 2020 at: https://www.pcimag.com/articles/87720-
improved-dirt-pickup-resistance-critical-to-future-
coating-innovation-1. - Khanjani J., Pazokifard S., Zohuriaan-Mehr, M.J.,
“Improving Dirt Pickup Resistance in Waterborne Coatings Using Latex Blends of Acrylic/PDMS Polymers,” Progress in Organic Coatings, 102(B), 151-166 (2017). - Khanjani J., Hanifpour A., Pazokifard S., Zohuriaan-Mehr M.J., “Waterborne Acrylic-Styrene/PDMS Coatings Formulated by Different Particle Sizes of PDMS Emulsions For Outdoor Applications,” Progress in Organic Coatings, 105267 (2020); accessed January 2020 at: https://www.sciencedirect.com/science/article/pii/S0300944019307337.
- Xu, F., Wang T., Bohling J., Maurice, A., Chen, H., Wu, L., Zhou, S., “Insight into the Dependence of Dirt
Adsorption/Desorption on the Surface Wetting Behavior Of TiO2–based Nanocomposite Coatings,” Progress in Organic Coatings, 131,137-144 (2019). - Sandoval, R., Bell, T., Hibben, M.J., “Exterior Durability in All-Acrylic Architectural Gloss Coatings: Gloss Retention and Dirt-Pickup Resistance,” Proceedings of the Annual International Waterborne, High-Solids, and Powder Coatings Symposium, 44, 49-58 (2017); accessed May 2020. https://www.epscca.com/opencms/export/sites/epscca/galleries/pdfs/articles/Waterborne_
Symposium_Paper_2017_Sandoval_Final.pdf. - Diebold, M.P., Kraiter, D.C., “Effect of Exposure Conditions on Dirt Pickup Resistance (DPR),”Journal of Coatings Technology and Research, 17 (3), 597-611 (2020).
- Saiz-Jimenez,C. “Air Pollution and Cultural Heritage,” Taylor & Francis Group, ISBN 905809 682 3. 2004.
- Mason, M., and Weaver W., “Settling of Small Particles in A Fluid; Mathematical Theory,” Phys. Rev. 23, 412 (1924).
- Hinds, W, Sioutas, C., Yifang, Zhu, Y. “2004 Progress Report: Relationship between Ultrafine Particle Size Distribution and Distance from Highways”. EPA Grant R827352C006 https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/6984/report/2004.
- US EPA. “Air Quality Criteria for Particulate Matter, Volume II of III”. EPA/600/P-95/001bF. 1996.
- Brown, S., Diebold, M., Kraiter, D., Jernakoff, P., Velez, C. Process and Apparatus for Quantifying Solid Residue on a Substrate, US Provisional Patent Applications 62/812543 and 62/933712. The Chemours Co.
- Examples: Mitina VK, Wolfgang P. Modified silica particles and dirt repellent polymer compositions comprising modified nanosilica. US 20100288963, US Patent and Trademark Agency (2010); Wolfgang P, Schacht H-T, Mitina VK, Kamatsch B, Powell K. Coating compositions with improved dirt pickup resistance comprising layered double hydroxide particles. US20110040006, US Patent and Trademark Agency (2011).
- Ragunathan K., Thakkar, P., “Novel Acrylic Polymer for Architectural Exterior Paint and Primer in One Coatings Applications,” CoatingsTech, 15 (2). (2018); accessed online January 2020. https://insights.basf.com/home/article/read/study-a-novel-acrylic-polymer-for-architectural-exterior-paint-and-primer-in-one
- Monaghan, G., “Formulating 50 VOC Gloss Floor Paint,” CoatingsTech, (6), 1-10 (2009)
- Zhou S., Yang, Y., Wang, T., Chen, H., Bohling, J., Maurice, A.M., Wu, L., “A Novel Adsorption Method To Simulate The Dirt Pickup Performance Of Organic Coatings,” Journal of Coatings Technology and Research, 15(1) 175-184 (2018).
CoatingsTech | Vol. 17, No. 6 | June 2020
