By Gloria Klees
Active ingredients such as oxybenzone and octinoxate have been used as major UV protectants in sunscreens for decades. While these ingredients are key components in many sunscreens, they create potentially harmful conditions to marine life.
Annually, an estimated 14,000 tons of sunscreen1 ends up in our oceans. Chemicals such as oxybenzone and octinoxate are threatening the health of aquatic organisms, specifically coral. When coral is exposed to these harsh chemicals they will expel zooxanthellae, microscopic algae that create food for the coral. As a result, the coral becomes bleached, leaving them more susceptible to disease. Many coastal areas have banned sunscreens2 containing these chemicals and officials have encouraged beachgoers to convert to mineral sunscreens.
Mineral sunscreens reflect the sun’s rays away from the skin unlike chemical sunscreens that use synthetic compounds to absorb the UV light. The objective of this experiment was to demonstrate how natural sun blockers can be an effective alternative to commercial chemical sunscreens that are harmful to sensitive environmental ecosystems such as coral reefs. The hypothesis was that if homemade sunscreens were created using nonharmful ingredients with natural sun protection factor (SPF), then they would be able to withstand UV rays.
While this experiment focuses on UV-ray protection in sunscreen, the results of this experiment can be transferred to numerous other materials, such as paints and coatings that require UV protection and their potential effective natural replacements.
To test the hypothesis, a variety of ingredients with differing SPF levels and materials were used to create homemade sunscreens and conduct the experiment. The three base ingredients were beeswax, shea butter, and coconut oil. While these have little to no SPF, they were used as vehicles for the other ingredients. The other ingredients used were raspberry seed oil, non-nano zinc oxide, carrot seed oil, jojoba oil, propolis extract, buriti oil, and strawberry extract. All have natural sun protection factors: some are high in SPF, such as carrot seed oil and zinc oxide, while others are low, such as buriti oil. Using these ingredients, six different recipes were created with different SPF levels based on the combination of ingredients.
Sunscreen A contained 4% carrot seed oil, 58% shea butter, and 38% coconut oil. Sunscreen A was made to test the effectiveness of a sunscreen containing only one high-SPF ingredient.
Sunscreen B contained 17% beeswax, 52% coconut oil, 17% jojoba oil, and 14% zinc oxide. Sunscreen B was mainly created to test zinc oxide’s effectiveness as the other ingredients all had low SPF.
Sunscreen C was made using 5% buriti oil, 5% propolis extract, 68% coconut oil, and 22% beeswax. This sunscreen was created to test the UV protection using only low-SPF ingredients. Sunscreen D contained 85% shea butter and 15% strawberry extract. The purpose of Sunscreen D was to test the lowest estimated SPF level made using the ingredients.
Sunscreen E was made up of 5% carrot seed oil, 20% zinc oxide, 50% shea butter, and 25% beeswax. Sunscreen E’s purpose was to test a homemade sunscreen using high-SPF ingredients.
Sunscreen F contained 7% raspberry seed oil, 26% zinc oxide, and 67% shea butter. Sunscreen F was also created to test a high-estimated-SPF sunscreen and to compare against Sunscreen E.
To create the sunscreens, all ingredients were melted down using a double boiler system and then manually mixed into their intended recipe. After mixing to homogeneity, the sunscreen was placed in a plastic container and left to rest and equilibrate overnight at room temperature. The sunscreens were tested the next day.
The materials used to test their effectiveness against UV rays were a reptile basking lamp containing UVA and a UVB light to mimic the sun, cardboard to act as the skin, SPOTMYUV UV stickers to test the effectiveness of the sunscreens, and a timer.
Because the sun emits UVA, UVB, and UVC rays, the basking lamp was used so the sunscreens would have equal levels of UV during each trial. The UV stickers used for testing begin as dark purple. When sunscreen is applied and exposed to UV rays, the sticker becomes clearer, demonstrating how effective the sunscreen is.
Figure 1 shows the UV stickers and light source prior to the sunscreen application and light exposure. If the sticker remains dark purple, that indicates that the sunscreen is not protecting the “skin” (cardboard) against the sun. These stickers were used to test the different sunscreens’ ability to withstand the UV rays when exposed to the basking lamp. To ensure an even application, a mini cosmetic spatula was used to spread a thin layer on each sticker. 
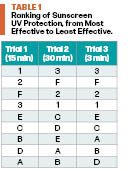
Three trials were conducted. Each varying in time, the trials tested the six homemade sunscreens alongside three store-bought sunscreens, which were labeled 1 through 3, that were used as comparisons. Trial 1 was 15 minutes with observations recorded every 5 minutes. Trial 2 was 30 minutes with observations every 10 minutes, and Trial 3 was one 5-minute observation. Figure 2 displays the results of Trial 2 after 30 minutes of UV exposure. Note that in Figure 2, lighter spots indicate better sun protection.

To rank the effectiveness of each sunscreen, the stickers were transferred to a white sheet of paper and ranked from lightest to darkest. After recording all the data from the trials, each trial’s sunscreens were ranked from most to least UV protection. Once all the data were collected, it was recorded into Table 1, which ranked each sunscreen’s effectiveness in each trial. The conclusion made from the rankings showed that the store-bought sunscreens were the most effective in protecting the skin against UV rays, but the homemade sunscreen F (raspberry seed oil, zinc oxide, and Shea butter) was only slightly less protective.
Overall, the results from the three trials partially support the hypothesis that homemade sunscreens, created with ingredients containing natural SPF, will be able to protect against UV rays. While homemade sunscreens such as D and B were unsuccessful in protecting skin against UV rays, others, such as Sunscreen F, were close to commercial-level sunscreens. The reason for this difference in effectiveness between the homemade sunscreens comes from the natural SPF levels of the ingredients used in each recipe.
From this experiment, one can conclude that high-SPF ingredient sunscreens can be effective in protecting the skin from UV while also helping to save the ocean’ coral reefs. This experiment can also be used to support other chemicals, possibly effective natural replacements.
References
- National Museum of Natural History. The Truth About Corals and Sunscreen. https://ocean.si.edu/ecosystems/coral-reefs/truth-about-corals-and-sunscreen#; September 2022. (Accessed October 25, 2023).
- Isabelle Côté. Beaches are banning sunscreens to save coral reefs. The Conversation. https://phys.org/news/2019-03-beaches-sunscreens-coral-reefs.html. Published March 5, 2019. (Accessed October 25, 2023).
View this article in the November-December 2023 digital issue of CoatingsTech.
