By Ronald T. Obie and Cameron Anderson, Wood Coatings Research Group, Inc.
Fast-curing acid-catalyzed alkyd-aminoplast coatings are often utilized as protective coatings for wood cabinet coatings. Quantitative assessment of impact of resin, crosslinker, and catalyst concentration on the curing process can be challenging for these and other coatings as well.
In the development of these coatings, curing is often assessed after the coating film has been exposed to a given cure profile by evaluation of physical properties such as hardness development as a function of time after exiting an oven. Curing under ambient conditions is often assessed by evaluating coating hardness development as a function of ambient drying-time conditions.
Evaluation of the influence of solvent on cure and crosslinking is often assessed by means of the finger-touch-down method as the sample is drying. Although these methods are very useful, they do not give quantitative insight into the drying and curing of the coating during the early drying process.
The current work investigates the effect of resin and crosslinker composition, and catalyst concentration, on the kinetics of curing of solvent-based acid catalyzed alkyd-aminoplast wood coatings. Further, the research investigates dynamic mechanical thermal properties of the resulting coatings after exposure to a given cure profile.
Introduction
Acid-catalyzed alkyd-aminoplast coatings for wood coatings applications are commonly based on alkyd resins crosslinked with urea- and/or melamine-formaldehyde crosslinkers and resins. Economical, “water white,” color stable varnishes typically consist of coconut fatty-acid-modified alkyd resin in combination with urea- and/or melamineformaldehyde crosslinkers. Curing at low temperatures is possible by the addition of a strong acid catalyst such as para-toluene sulfonic acid (PTSA). Rate of cure, development of physical properties, and long-term durability of these varnishes is dependent upon a variety of factors, such as alkyd resin chemistry, crosslinker composition, solvent selection, and catalyst chemistry and concentration.
Alkyd resins may vary in oil type and length, hydroxyl number and its reactivity (i.e, primary, secondary, etc.), molecular weight, molecular weight distribution, and degree of branching, as well as other parameters.

Assessment of Curing
Most practical studies relating to assessment of curing of alkyd-aminoplast coatings typically involve evaluation of hardness development of the cured film as a function of time, temperature, catalyst level, or some combination of these parameters.
Such tests include indentation hardness tests as described in ASTM D 1474,3 (e.g., Knoop and Pfund hardness numbers); instrumented indentation testing such as those described in ASTM E2546-15;4 pendulum damping tests such as those described in ASTM D 43665 (e.g., König and Persoz Pendulum Hardness Tests); and scratch hardness test such as those performed with pencil, as in ASTM D3353.6 All these test methods require the coating to have reached some degree of cure and/or hardness development before hardness may be assessed, thus losing the ability to assess cure of the film in its sol state.
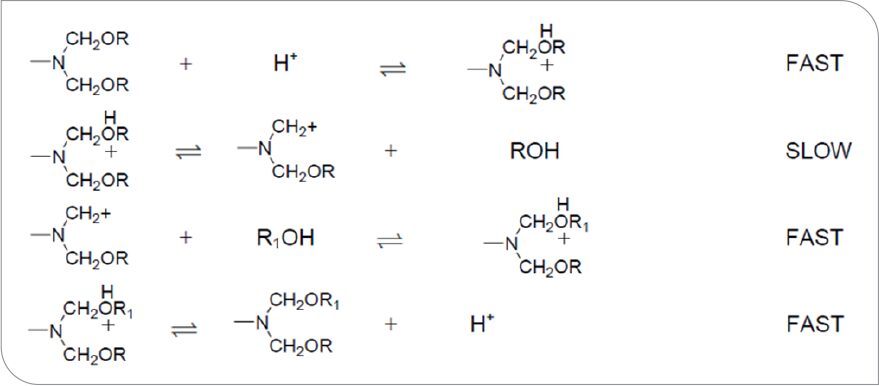
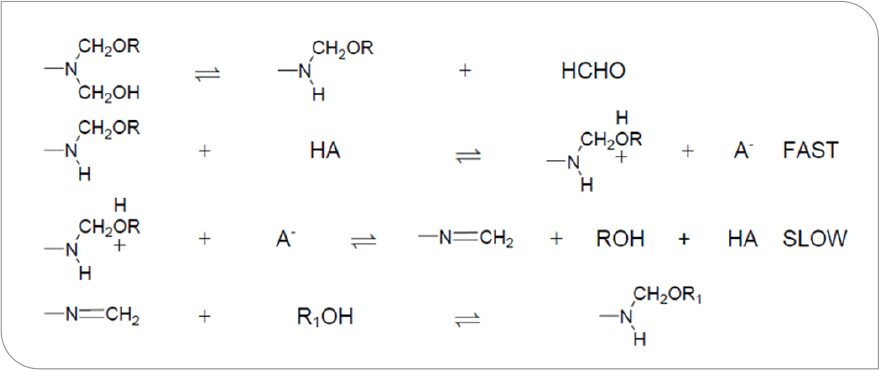
Utilizing gas chromatography to analyze reaction volatiles in polyester coatings crosslinked with various melamine formaldehyde crosslinkers, Blank7 states that the loss of alcohol from the film by diffusion and/or evaporation has a significant influence on reaction rate of the coating. It is common practice when formulating alkyd-aminoplast coatings to utilize judicious blends and concentrations of alcohols as solvents to help control coating pot life, the rate of flash off, lay open time, and cure rate of the composition. The compositions studied by Blank were typically cured at temperatures of 100 °C or higher for about 20 minutes. He did not look at the influence of variables on cure at early times nor the impact of solvent composition. Blank proposed a specific acid catalysis mechanism for highly alkylated melamine formaldehyde resins and a general acid catalysis mechanism for partially alkylated resins and/or fully alkylated, high-imino resins. The reaction schemes proposed by Blank for these two cure mechanisms are shown in Figures 1 and 2, respectively.