By Wumin Yu, Ingevity Corporation USA
Biobased materials are increasingly important in the coatings industry as more companies align their sustainability goals to reduce environmental footprints and develop more eco-friendly products to meet customer needs. However, widespread adoption of biobased materials in coating applications can be challenging due to the lack of available biobased materials that are both cost-effective and performance competitive.
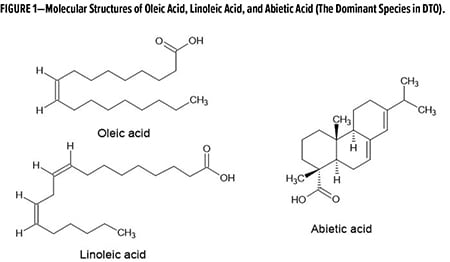 To solve these challenges, we developed a series of cost-competitive biobased epoxy resins using distilled tall oil (DTO) as the feedstock. DTO, a bio-refinery product derived from crude tall oil, is a byproduct of the pinewood pulping process and is 100% biobased. DTO is a mixture of tall oil fatty acids (TOFA)—mainly oleic acid and linoleic acid—and tall oil rosin acids (rosin)—mainly abietic acid and its isomers. Figure 1 shows the molecular structures of the main components in DTO.
To solve these challenges, we developed a series of cost-competitive biobased epoxy resins using distilled tall oil (DTO) as the feedstock. DTO, a bio-refinery product derived from crude tall oil, is a byproduct of the pinewood pulping process and is 100% biobased. DTO is a mixture of tall oil fatty acids (TOFA)—mainly oleic acid and linoleic acid—and tall oil rosin acids (rosin)—mainly abietic acid and its isomers. Figure 1 shows the molecular structures of the main components in DTO.
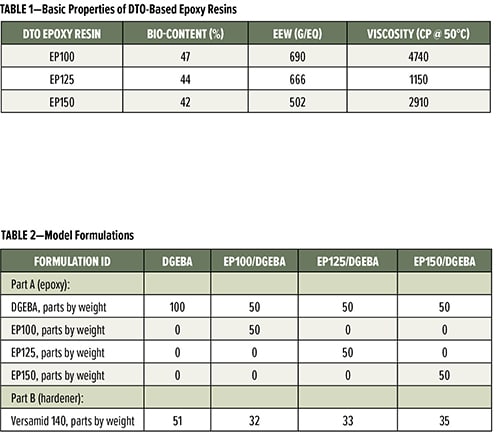
The properties of these novel DTO-based epoxy resins can be tuned by changing the ratio of TOFA to rosin in DTO. The three DTO-based epoxy resins listed in Table 1 are amber color liquids at room temperature, have a biocontent range of 40% to 50% and an epoxy equivalent weight (EEW) ranging from 500 to 700 g/eq. They are designed for use in applications such as coatings, composites and adhesives, and performance enhancement(s) can be achieved with proper formulation. This article will demonstrate how these novel DTO-based epoxy resins can help improve miscibility and compatibility, water resistance, adhesion, flexibility, impact resistance, and chemical resistance in 2K epoxy coatings.
EXPERIMENTAL SECTION
Coating model formulations
The 2K epoxy coating formulations for this study are listed in Table 2. The name of each formulation represents the combination of the epoxy resins used. For example, the first formulation that only contains diglycidyl ether of bisphenol A (DGEBA) (EEW = 187 g/eq) in its epoxy part is named as DGEBA and used as the control. The other three formulations, EP100/DGEBA, EP125/DGEBA and EP150/DGEBA, all have a mixture of 50% by weight of DGEBA and 50% by weight of one of the DTO-based epoxy resins listed in Table 1. The amine hardener used in this study is Versamid 140, a polyamidoamine hardener from Gabriel. In each formulation, the ratio of epoxy to amine hardener was kept at 1:1 equivalent ratio.
Characterization
The mixing study was conducted at room temperature (23 ± 2 °C) on a 150g formulation scale with a mechanical stirrer rotating at 300 rpm. For the curing behavior study, 150g of Part A (epoxy) and Part B (hardener) mixture in a plastic cup was placed in a 25 °C water bath and the viscosity and temperature of the mixture were closely monitored to obtain the gel time and exothermic peak temperature. The viscosity of the mixture was monitored by a Brookfield CAP 2000+ viscometer and the point at which the viscosity reached the maximum limit with a #03 cone spindle at 50 rpm and 50 °C was defined as the gel time.
For tensile, dynamic mechanical analysis (DMA), Shore D hardness and water absorption test samples, the formulation mixtures were first cured in silicon molding at room temperature overnight and then removed from the silicon mold and post-cured at 100 °C for two hours. The tensile test was performed on an Instron 3365 universal testing machine at a crosshead speed of 10 mm/minute according to ASTM D638.
The DMA test was conducted using a 3-point bending geometry on a TA Instruments DHR-2 rheometer at a heating rate of 2 °C/minute and the tan delta curve maximum was used as the glass transition temperature (Tg) of each cured sample. The Shore D hardness was measured with a Shore D durometer.
The water absorption test was carried out by immersing a cured sample (44mm x 13mm x 3mm in dimension) in water at room temperature. Every three or seven days, the sample was taken out and water on the sample surface was wiped off before measuring the weight gain. The water absorption percentage after t days of water immersion was calculated as: ![]()
where M0 and Mt are the initial sample weight and the sample weight after t days of water immersion, respectively.
The 2K epoxy formulations listed in Table 2 were coated onto aluminum (type A, 3003 H14 alloy, smooth mill) and steel (type R, cold-rolled steel, dull matte) Q-panels using a 6-mil drawdown bar for coating-performance evaluation. The coating dry time was recorded with a GARDCO DT-5040 quadracycle electronic dry time recorder (ASTM D5895) on a 3-mil wet film drawn down on a Lenata 2A opacity chart. All Q-panels were cleaned with acetone and dried for 15 minutes before applying coatings.
The coated panels were kept at room temperature to cure for seven days with some coated panels going through an additional two hours of post-cure at 100 °C if needed before characterization. The dry film thickness was roughly 3 mils. The coating flexibility was measured with a TQC mandrel bend tester (ASTM D522). Impact-resistance measurements were performed with a BYK Gardner 1102 impact tester (ASTM D2794).
The tape test method (ASTM D3359) was used for coating adhesion evaluation. The chemical resistance of each coating sample was evaluated with a spot test method. Nearly one milliliter of each of the testing chemicals was placed on the surface of a coated panel and covered with a watch glass. Each chemical droplet was kept in contact with the panel for 24 hours and then the contact area was examined for damage after the residual chemical was wiped off.
