By Jonathan Doll, Michael Venturini, and Anthony Rohrer, Sun Chemical Corporation
Introduction
Powder coatings are solvent-free coating systems used for many applications due to their durability, ease of application, and environmental advantages vs liquid coating systems. Powder paints are applied to a substrate electrostatically and cured at relatively high temperatures to form a continuous, durable film. The advantages of powder coatings are that no solvents are used when they are sprayed, minimizing environmental issues related to volatile organic compounds (VOCs). Additionally, any material that does not adhere to the substrate after spraying, known as “overspray,” can be collected and used again, resulting in very little waste.
Powder coatings are typically made by combining resins, a crosslinker, fillers, organic or inorganic absorption pigments, and additives together in an extruder. The resin is melted, and the ingredients are intensely mixed into the resin. The extruded resin is collected and milled to a fine particle size distribution to then be sprayed.
Effect pigments owe a significant portion of their color and appearance to their structure and shape. In the case of aluminum pigments, a silver to gray metallic appearance is observed due to their shape. Most aluminum pigments are micron sized platelets with large aspect ratios (pigment width/pigment height). In an application, aluminum pigments behave like “micro-mirrors,” reflecting nearly all the light that interacts with them in a specular or near-specular angle. These pigments display the so-called “flip-flop” effect, which is a light to dark color travel when viewing a display head-on vs at a diffuse angle.
Additionally, aluminum pigments have a bright, silvery appearance that is defined by their brightness (L*) values and gloss. Platelet-shaped aluminum pigments have excellent hiding power when compared to spherical pigments. Because of their shape, they are very susceptible to bending and breakage under high shear mixing, like extrusion, and are typically incorporated into powder coatings in subsequent processing steps.
Metallic pigments can be dry blended into a powder coating. However, because this only makes a physical mixture between two powders, this can cause issues during application of the powder coating, where the pigment and coating particles separate due to electrostatically charging differences. This causes the overspray to be unusable due to inconsistent color.
To mitigate these issues, a process called bonding is often used to incorporate metallic pigments into a powder coating. During bonding, the powder coating is heated to just above its softening point and combined with effect pigments under moderate shear, causing the pigments to adhere to the surface of the powder coating. Such a process provides sprayed parts that have excellent pigment orientation, resulting in the best appearance for powder-
coated articles.
In addition to their shape, aluminum pigments have certain chemical properties that impact their orientation in powder coatings. Aluminum pigments are typically made by ball milling a three-component system of aluminum grit, hydrocarbon solvent, and lubricant. For powder coating applications, the solvent is removed, but the lubricant stays behind on the surface of the pigment. Typical lubricants are saturated and unsaturated analogues of C18 fatty acids, namely stearic and oleic acid, respectively.
When saturated fatty acids are used, the aluminum pigments will have a low surface energy, causing them to rise to the air-coating interface. The pigments crowd the surface and have superior alignment in the coating, resulting in a minimization of scattering centers and a highly glossy appearance. These types of pigments are known as leafing. When unsaturated fatty acids are used, the pigment surface is wetted by the coating, and the pigments are evenly distributed through the thickness of the coating, giving a duller metallic appearance known as nonleafing behavior.
This behavior can have a profound impact on the appearance of two otherwise identical pigments. For example, a nonleafing cornflake will have lower brightness, flop, and gloss when compared to its leafing analogue. Moreover, the hiding power of the leafing pigment will be higher, resulting in reduced pigment loading requirements for nonleafing pigments. Leafing pigments have the drawback that, since most of the pigment is located on the surface of a coating, they are more susceptible to scuffs and staining than nonleafing pigments.
In powder coatings, a solvent-free effect pigment powder must be used, which can cause a large release of dust as it is being processed and handled. For metallic pigments, the dust not only poses an inhalation hazard, but is potentially explosive if clouds of dust are generated. This risk increases with the fineness of the pigments and requires special safety precautions to mitigate.
It is well known in the powder coating industry that aluminum pigments need to be handled with care as the coupled effect of small size and reactivity mean that aluminum pigments have a low minimum ignition energy (MIE) and high kST. The MIE and kST are measures of how much energy is required to ignite a powder or dust and the strength of that explosion, respectively. It is typically a function of the particle size of the dust. Dry, powdered aluminum pigments, which can have a d50 of as low as 5 µm, have a low MIE (<5 mJ) and high kST (>300 bar m/s).1 These hazards increase as the size of the aluminum pigments decrease.
In other industries where aluminum needs are used in a solvent-free form (e.g., plastic masterbatch), aluminum is often combined with a resin, extruded, and dried to make a pelletized product. The pellet mitigates dust, increasing the MIE and decreasing the likelihood of an explosion. If this approach could be applied to an aluminum pigment intended for use in powder coating, it could provide a benefit to converters who need to work with fine aluminum pigments. For such a preparation to work, however, the pellet would need to be easily friable and dispersible in a powder coating while the compounding resin would need to be widely compatible with many different powder coating types.
In this article, a pelletized aluminum preparation for powder coatings is presented that combines a fine (d50 = 9 µm), leafing aluminum pigment with a polyester resin. These products have an aluminum content of 85%, with the remaining fraction being the resin, which allows formulators to use these products without having a dramatic impact on the resin content of the base paint. The pellet is highly friable and can be incorporated into most polyester-based powder coatings by either high shear dry blending or bonding to give an appearance that is like a bonded aluminum powder of equivalent size and composition. A description of the test methods used and results in polyester and other resin chemistries are presented.
Materials and Methods
Description of Aluminum Pellets
In the following examples, the pelletized aluminum pigments are made using a fine, leafing corn flake pigment with a d50 particle size distribution of 9 mm. They are composed of approximately 85% aluminum flake and 15% of a polyester resin. The pellets are dry and approximately 5 mm in diameter, as seen in Figure 1.

Incorporation into Powder Coating
The pelletized pigments can be incorporated into powder coating by two different methods: high shear dry blending or bonding. For high shear dry blending, the pellets are added to a powder coating base at 1.25% total metal loading and mixed at 50% of the speed/time for a typical bonding step. These samples were compared to a dry-blended leafing aluminum (Benda-Lutz Leafing 2081) that was incorporated under the same conditions and at the same loading. Bonding of the pelletized and unpelletized aluminum was carried out at 1% total metal loading using standard bonding protocols. The pigmented powder coating bases were applied to stainless steel panels and cured according to the parameters of the base.
The 60o gloss, brightness (L*15, measured at 45o incident light and 15o aspecular) and the flop index (FI), which is determined by equation (1):
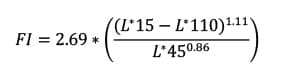 where L*45 and L*110 are brightness measured at 45o incident light and 45o and 110o aspecular reflection angles, respectively. All color measurements were performed on an X-Rite MA-98 multiangle spectrophotometer. Gloss measurements were performed on an Elcometer 6015 Novo-Gloss IQ Glossmeter.
where L*45 and L*110 are brightness measured at 45o incident light and 45o and 110o aspecular reflection angles, respectively. All color measurements were performed on an X-Rite MA-98 multiangle spectrophotometer. Gloss measurements were performed on an Elcometer 6015 Novo-Gloss IQ Glossmeter.
Results & Discussion
Dust Testing
The MIE of a dust cloud is the lowest energy discharge required to ignite the dust/air mixture at room temperature. Table 1 shows the MIE for a fine aluminum pigment and a 2 mm diameter pellet made using the same. Modification of the pigment’s physical form is one of the few ways to raise the MIE as it allows for a decrease in dust concentration and significantly larger suspended particles. In standard MIE testing, only particles less than 75 mm are tested. The pelletized product has increased the MIE of the fine aluminum powder by two orders of magnitude, which is a substantial change. Further increases in the pellet’s size are expected to yield a larger MIE value.
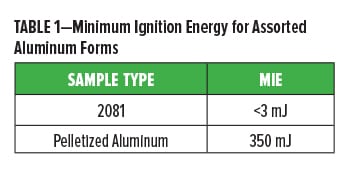
This data is important as it affects the transportation and storage requirements for different forms of aluminum pigments. Aluminum pastes, powders, and pellets fall into specific categories for transportation and storage in each region depending on local regulations. Because of their reactive nature, aluminum powders are classified in the United States by the Department of Homeland Security as “Chemicals of Interest.” As such, there are specific guidelines depending on quantities to ensure against their misuse. There are also specific guidelines for transportation set by the various agencies in North America and Europe. Aluminum powders can be classified as hazardous materials by the U.S. Department of Transportation (DOT), or dangerous goods under the European Agreements Concerning International Carriage of Dangerous Goods by Road and Rail (ADR/RID). In China, Decree 591 sets guidelines specifying certified warehouses for storage of aluminum powders.
Pellets, however, and specifically pellets designed for powder coatings, are exempt and not regulated as dangerous goods per the applicable hazardous material transportation regulations. This can significantly simplify the supply chain and logistics for using pellets as compared to aluminum powders.
Another smaller consideration that is growing in importance is packaging materials. Aluminum powders and pastes are commonly packaged in steel drums while pellets are packaged in a bag-in-carton containers. Steel drums can be problematic for some manufacturers to recycle by introducing another waste stream while bag-in-cartons neatly fit into warehouse racking systems and can be easily recycled.
Appearance Testing
Figure 2 shows the L*15 (a, d, and g), flop index (b, e, and h), and brightness (c, f, and i) of the pelletized aluminum and the leafing aluminum control (2081) after being incorporated into either a triglycidyl isocyanurate (TGIC)-cured (a–c) or a b-hydroxyalkyl-amide (HAA)-cured (d–f) polyester powder coating. Figure 2g–i shows the results for the aluminum pellet in a polyurethane-type of powder coating. In addition to a comparison of the different forms of aluminum, Figure 2 also shows the different appearances that can be achieved depending on the incorporation method used, whether it is high-shear dry blending (red and blue bars) or bonding (green and yellow bars).
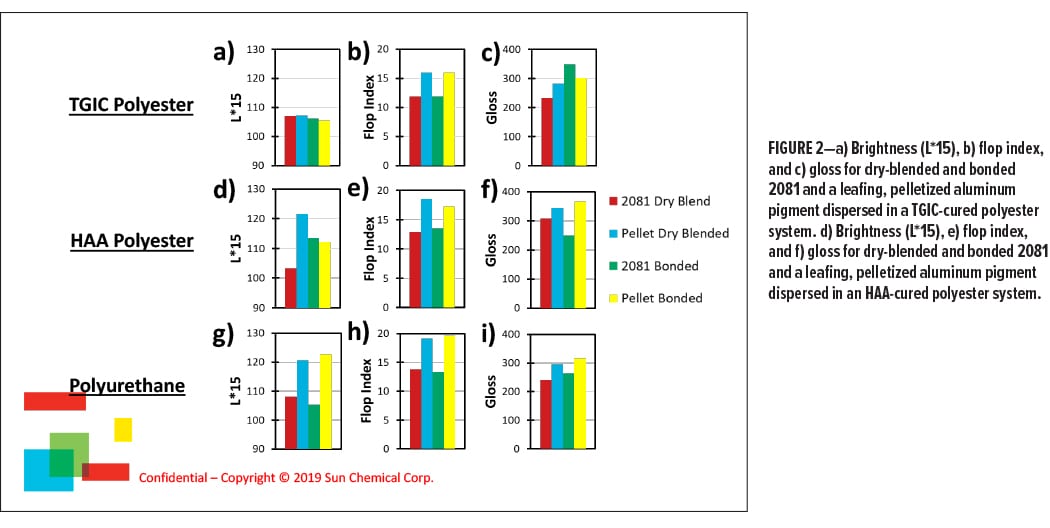
Focusing on the TGIC examples first (Figure 2a–c), reveals that the pelletized aluminum pigment has a similar brightness to the 2081 in this system. More surprising is that, in this system, the dry blended and bonded pellets are superior in flop compared to blended or bonded, unpelletized aluminum. The dry blended and bonded pellets have similar gloss, and both are less glossy (Figure 2c) than the bonded aluminum. In all cases, the dry blended leafing pigment is the most inferior in flop and gloss for the TGIC-cured polyester.
This data shows that the pelletized aluminum can be incorporated by two different methods to give a bright metallic finish that is comparable to the bonded, loose aluminum. Moreover, the flop data suggest that the pellets have a better overall orientation in this powder coating compared to unpelletized pigments. Indeed, the inclusion of the resin may help to pull the aluminum into a flat configuration when the powder coating is cured.
The gloss is best for the bonded aluminum, with the bonded, pelletized aluminum coming in second. This is not particularly surprising considering that gloss in powder coatings can be driven by the resins in the system. Depending on the resin compatibility, this could result in a smoother finish that is aided by the superior orientation of the aluminum, increasing the gloss.
If this is the case, it is not unreasonable to expect that the pelletized aluminum may exhibit different types of behavior in different resin systems and classes. The dry-blended aluminum has the worst gloss, even when compared to the dry-blended pellet. This suggests that the pelletized aluminum may be better wetted out by the resin in the powder coating, which helps to drive its alignment.
The difference in incorporation behavior is demonstrated for the HAA-cured polyester shown in Figure 2d–f. In this instance, the dry-blended pellet has the higher L*15 and flop index, while the bonded pellet has the highest gloss. The pelletized pigments outperform the pigment powders, regardless of their incorporation method. This could be due to a similar phenomenon above, where the pellet resin positively affects the interaction between the aluminum and the powder coating matrix, acting almost like a dispersant.
Finally, Figure 2g–i shows the incorporation behavior in a polyurethane-based powder coating. In this instance, the pelletized aluminum is optically superior to the unpelletized aluminum in every case, even when comparing a dry-blended pellet to the bonded, unpelletized pigment.
While this data shows that the pelletized aluminums are compatible with different powder coating systems, it is likely that formulators would need to optimize performance of these pellets for a particular powder coating system.
Reclaim Testing
To test for the ability to reclaim a powder coating, a cyclone test was performed that mimics the reclaim for a large production. Cyclone separators use an airstream to separate the finer, lighter particles from a powder based on their weight and density. This test assesses whether a powder will separate when it is reclaimed.
Based on the good results from Figure 2 for the blended pellet, a test on the reclaim for powder coating containing this product was completed and compared to blended and bonded 2081 aluminum. Powder coatings containing each of the samples (in fact, the same powder coatings used in Figure 2a–c), were passed through a cyclone apparatus and a panel was sprayed and evaluated to give “Pass 1.” The process was repeated on the Pass 1 material to give Pass 2.
The results of the cyclone test are shown in Figure 3, which shows how the brightness (L*, Figure 3a), flop (Figure 3b), and gloss (Figure 3c) change with repeated cyclone cycles.
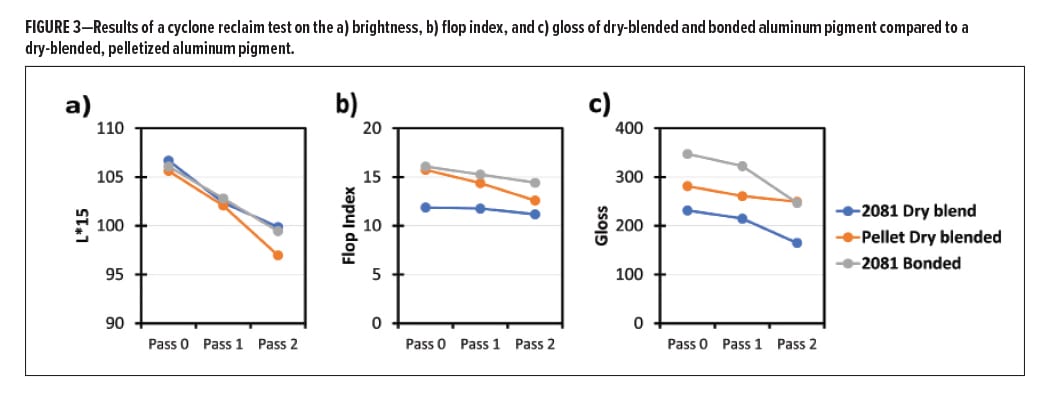
It is observed that upon repeated cyclone cycles, the brightness, flop, and gloss all are reduced. An interesting feature is the blended and bonded metallic pigment appear to change according to the same slope in all aspects, while the blended, pelletized aluminum has different behavior. This is unexpected, but not entirely surprising considering that there is an additional resin in these samples. The pelletized product shows a faster decline in brightness and flop, but an intermediate decline in gloss when compared to the unpelletized products. It is unclear as to why this is, but it may be due to the presence of the secondary resin and whether this drives different separation behavior.
In all instances, there was a visible degradation of the finish of the powder coatings. Under visible observation, the blended metallic pigment showed the least metallic finish, while the bonded metallic and the blended pellet had similar metallic effects. Finally, it is worth noting that this method does not test whether a powder will electrostatically separate when it is sprayed (transfer efficiency).
Conclusions
Powder coatings are a well-established paint technology, delivering many performance, economic, and environmental advantages. Aluminum flakes are widely used in all coating types because of their brightness, opacity, durability, and formulation flexibility. Aluminum flakes, however, require specific handling and storage techniques to maximize their appearance and for safe usage. Aluminum pellets are an alternative delivery form that can be incorporated using existing methods of dry blending or bonding to give equal or better appearance in a variety of paint chemistries. Pellets maintain the versatility and appearance benefits of aluminum powder while offering lower dusting and significantly higher MIE. The combined benefits and versatility of pellets make them an attractive option in powder paint manufacturing.
References
- OSHA 3371-08 2009 “Hazard Communication Guidance of Combustible Dusts.”
CoatingsTech | Vol. 16, No. 7 | July 2019
