By Kyle Flack, Nicholas Foley, Tyrone Vaughn, Lisa Kicklighter, and John Mangano, BASF Corporation
Proper film formation is a critical factor in high performance coatings as it ensures optimally low porosity, improves corrosion resistance, and impacts many film surface features. Through the decades, waterborne coatings have relied on volatile coalescing aids (solvents) to enable hydrophobic latex particles to coalesce. Coalescing aids can be eliminated if the given latex has a glass transition temperature (Tg) below or near intended application temperature, but often the final paint properties suffer due to the increased softness of the final film. Therefore, harder latexes have been developed, which require assistance from a coalescing aid to soften the system enough to form a continuous film during water evaporation (Figure 1). Coalescents aid in steps 3 to 4 of the drying process.
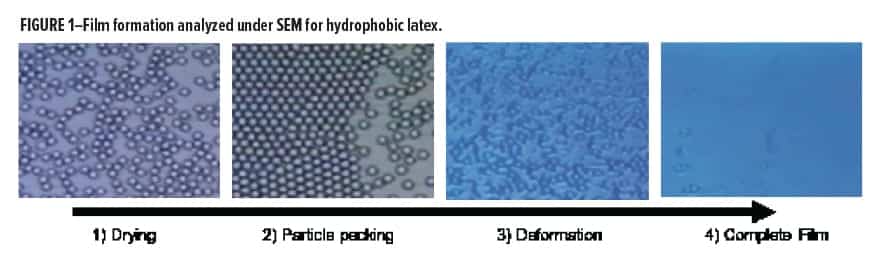 Historically, volatile solvents such as dipropylene glycol n-butyl ether (DPnB) or 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (Eastman Texanol™ Ester solvent, referred to as Texanol in the text), can be tuned based on compatibility with the given formulation and latex system to provide optimum film coalescence while remaining volatile during or after film formation to maximize filmic properties. The challenge for the industry has been regulation on volatile organic compound (VOC) levels allowed, especially in interior architectural coatings, with a drive towards elimination of all VOCs from a coating. To balance the requirement of limiting VOC content, concessions in the amount of volatile coalescent are often used. To account for the reduction in volatile coalescent loading, permanent or nonvolatile coalescents have become of utmost importance. These materials interact in a similar way to their volatile counterparts while remaining solvated in the final dry film. This permanence can have some drawbacks, namely, in secondary paint properties, such as poorer block resistance and increased leaching.
Historically, volatile solvents such as dipropylene glycol n-butyl ether (DPnB) or 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (Eastman Texanol™ Ester solvent, referred to as Texanol in the text), can be tuned based on compatibility with the given formulation and latex system to provide optimum film coalescence while remaining volatile during or after film formation to maximize filmic properties. The challenge for the industry has been regulation on volatile organic compound (VOC) levels allowed, especially in interior architectural coatings, with a drive towards elimination of all VOCs from a coating. To balance the requirement of limiting VOC content, concessions in the amount of volatile coalescent are often used. To account for the reduction in volatile coalescent loading, permanent or nonvolatile coalescents have become of utmost importance. These materials interact in a similar way to their volatile counterparts while remaining solvated in the final dry film. This permanence can have some drawbacks, namely, in secondary paint properties, such as poorer block resistance and increased leaching.
Many options have become relevant in the marketspace for low- to zero-VOC coatings. Therefore, the goal of this work was to find optimum levels of coalescent required in a given system to understand the impact that an ultra-low VOC coalescent structure can have on efficiency and performance properties, and to investigate the interactions behind why one coalescent is preferred over another.
Experimental Design
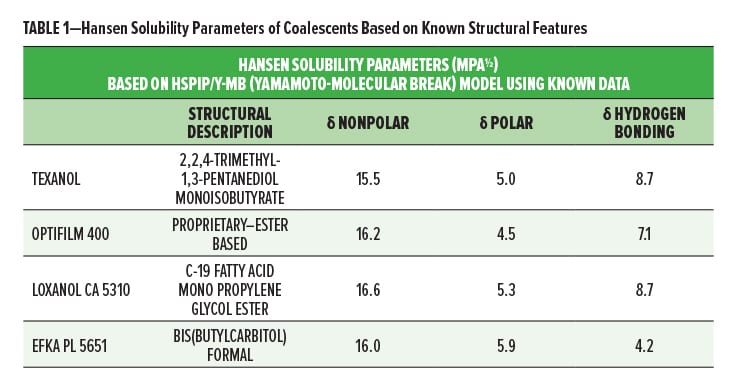
Evaluation of coalescent space was focused on three primary coalescing agents: Loxanol® CA 5310 and Efka® PL 5651 from BASF Corporation and Eastman Optifilm™ Enhancer 400 (from Eastman Chemical Company, referred to as Optifilm 400 for further discussion). These materials differ quite significantly in structure and, therefore, it would serve to understand the effect of different structures on the primary coalescing and secondary performance properties in various latexes. Optifilm 400 is a leading industry benchmark for ultra-low VOC coalescing agents, and Loxanol CA 5310 and Efka PL 5651 are also ultra-low VOC coalescing agents. Descriptors of the chemistries are shown in Table 1, along with the Hansen solubility parameter predictions.
Hansen solubility parameters were estimated and may be different than reported in literature based on the Y-MB model within the HSPiP software tool (version 3.1.14). Although some assumptions are made within the model, directional fits were consistent with literature reported values for Texanol and Optifilm 400, which gave indications that, as a comparative tool, these predictions are relevant.
To gain an understanding into coalescing efficiency, minimum film formation temperature (MFFT) was evaluated upon adding coalescing agents to diluted latex systems. The latex systems that were evaluated are described as Latex A, B, and C, and will be denoted as such throughout this text.
Latex A—All-acrylic latex designed for zero-VOC capable paints and enhanced cleanability
Latex B—All-acrylic latex for interior/exterior “paint and primer in one” systems
Latex C—Styrene/acrylic latex for primer applications
Influence of latex parameters such as particle size was also evaluated. Latex A was synthesized via emulsion polymerization maintaining the surfactant level in all cases and only modifying the number of particles accessible through polymer growth. This in turn allowed the variance of particle size from 99 nm to 135 nm, based on volume. Although one might expect the influence of such a small change to be minimal, we have shown that, in fact, the demand for coalescent can change.
Efficiency tests were conducted by adding 90 g of latex to a container, adding the appropriate level of coalescent to be tested, and adjusting with water to 100 g total. Samples were mixed under low shear paddle mixing for five minutes and then allowed to rest in a controlled temperature and humidity room (CTH, 50% relative humidity and 70°F) overnight.
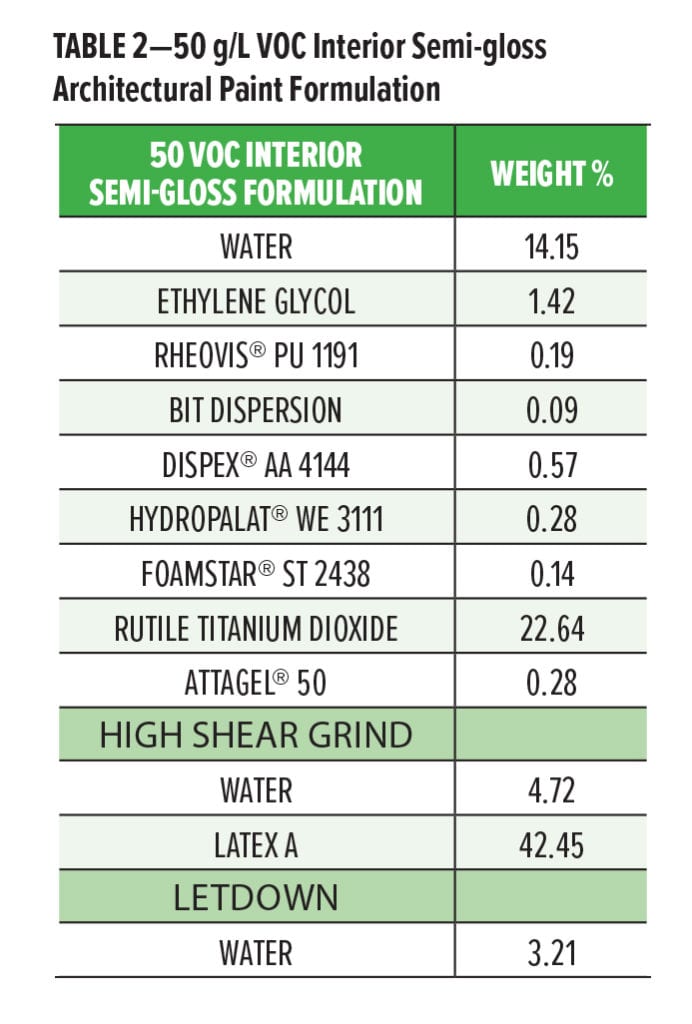
Paint evaluation was conducted in an interior architectural formulation with Latex A as the latex of study, shown in Table 2. Evaluations of the different coalescing aids were done in a full formulation and compared at equal loadings of coalescent across the systems. At the same loading, evaluation of some key performance parameters including gloss, scrub cycles, stain resistance (cleanability), and hardness were performed. Gloss measurements were conducted using a 7 mil Dow Film Caster drawdown bar on a sealed chart. The film was allowed to dry for 24 hours under CTH conditions before being read in triplicate with a BYK-Gardner micro-TRI-gloss meter. Scrub and stain resistance measurements were conducted by drawing down the control and test paint side by side with a 7 mil Dow Film Caster bar on a black vinyl chart. The drawdowns were allowed to cure for seven days under CTH conditions and were split in two. Half of the chart was used to analyze for scrub resistance and half was used to analyze for stain resistance.
Scrub resistance was conducted using a calibrated Gardco® * scrub machine fitted with brushes, using 10 mL aliquots of Leneta SC-2 abrasive medium, and 5 mL of water being added after each set of 400 cycles. Samples were evaluated as failure when a line due to complete film erosion (showing vinyl chart) was seen.
Stain resistance was completed by applying one-inch wide strips of stains of interest across the control and test paint, and allowing them to rest for one hour. The stains were then removed via a simple rinse to eliminate excess. The panels were placed on the Gardco scrub machine and the brush replaced with a sponge. Twenty-five cycles were run using household cleaner Fantastik® † as a cleaning agent. Pendulum (Koenig) hardness was conducted by drawing paints down at 10 mil (250 microns) on glass plates. The films were allowed to dry under CTH conditions for three days before conducting the pendulum swing test in duplicates.
*Gardco® is a registered trademark of the Paul N. Gardner Company, Inc.
†Fantastik® is a registered trademark of S.C Johnson & Son, Inc.
Results
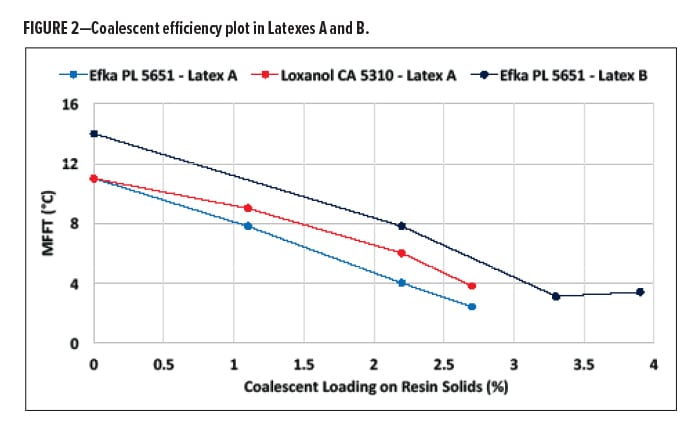
Initial exploration focused on the incorporation of the two coalescents from BASF, Loxanol CA 5310 and Efka PL 5651. The molecular weights of these materials are similar, although contributions from the polar and hydrogen bonding components are significantly different. Latex A was found to have suitable compatibility at all levels of testing for the Loxanol CA 5310 and Efka PL 5651. It was found that MFFT dropped more significantly with the Efka PL 5651 compared with Loxanol CA 5310 (Figure 2).
In Latex B, the two coalescents were also compared, and it was realized that incorporation into the latex system was surprisingly not manageable with Loxanol CA 5310. However, samples made with Efka PL 5651 provided clean, clear films at temperatures as low as 4°C. Very poor compatibility was found upon letting the sample rest. A layer of coalescent was found on the top of the samples that contained the Loxanol CA 5310. Even though the contribution based on solubility parameters would predict a higher hydrogen bonding and polar component over the Efka PL 5651, solubility in water is a limiting factor in the Latex B system. Multiple factors could be responsible for this differentiation. The mobility of the coalescent into the water phase enough to interact with the stabilized latex particles could be a contributing factor, although with Latex A, we observed stable systems with both coalescents. This observation would suggest that perhaps the stabilizing surfactants in Latex B are significantly altering the interaction or miscibility of the coalescent with the latex.
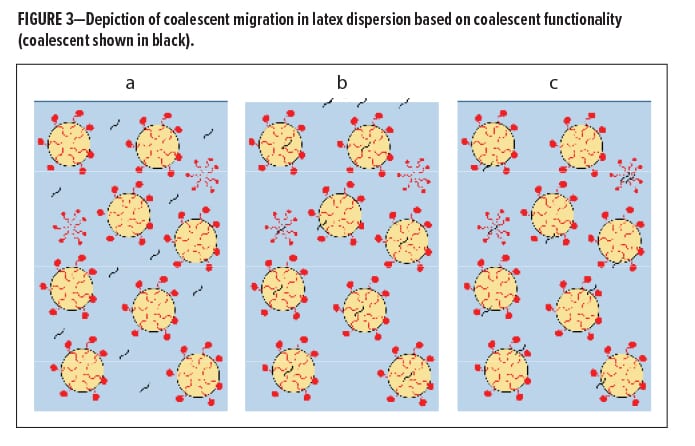
Figure 3 shows the case where (a) a coalescing aid (in black) is highly water soluble and, therefore, quite ineffective due to decreased interaction with hydrophobic latex species, (b) a coalescing aid is very latex soluble but has very low water solubility, and (c) where the coalescing aid is surfactant miscible and stable at the boundaries of the latex particles. An explanation for the lower efficiency of the Loxanol CA 5310 compared to Efka PL 5651 is that the Loxanol CA 5310, being very surfactant-like in structure, is on the edge of compatibility with the hydrophobic latex and being surfactant-like itself, leads to poor migration through the water phase to interact with the latex particles. If it can form its own micelles, that could potentially lead to reduced efficiency.
A visualization of the incompatibility can be seen in Figure 4. MFFT could not be recorded based on the creeping and film issues seen when Loxanol CA 5310 was used in Latex B (due to immiscibility).
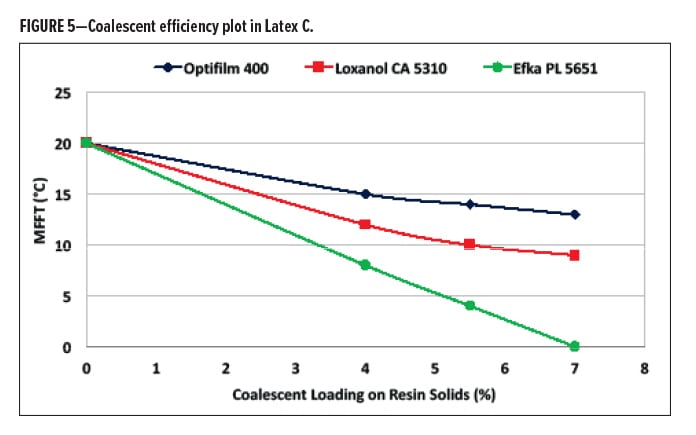
Coalescents were also compared in Latex C, a styrene-acrylic (Figure 5). In this particular case, Optifilm 400 was also introduced as a coalescing aid with distinctly different contributions for polar and hydrogen bonding factors. In conducting the loading study of the three coalescing aids in this system, it was found that the order of efficiency was Efka PL 5651, Loxanol CA 5310, followed by Optifilm 400. Loadings were evaluated to reach an MFFT of 0°C. With an MFFT of 20°C of noncoalesced latex, effectively at 7% based on latex solids, Efka PL 5651 showed a marked increase in efficiency comparatively. All systems at these loadings were found to be stable and miscible.
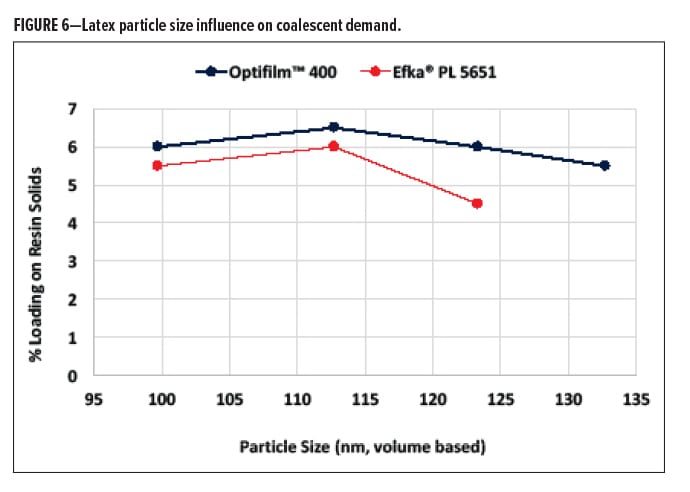
To further understand the role of latex composition of coalescent selection, particle size was taken into account. Because spherical particles’ surface area is calculated through a squared function, small changes in particle size result in large changes in effective surface area. We evaluated this as a function of the two coalescents, Efka PL 5651 and Optifilm 400. It was found that efficiency differences were noted, as suggested previously, based on the difference in solubility contributions. Interestingly, it was realized that both coalescents were a effected by particle size changes within a given particle size range. Particle sizes were compared between 99 nm and 135 nm where a trend was noticed (Figure 6). As particle size was increased from 99 nm to 112 nm, a slight decrease in coalescent efficiency was realized.
Increasing the particle size from 112 nm to 135 nm showed a substantial slope change, indicating that plasticization of the given latex system was affected positively upon decreasing surface area. The same amount of surfactant was dosed into the synthesis of the latex, as surface area decreased (particle size increased), more free surfactant would exist in the water phase, either acting as a shuttle to carry the coalescent more effectively to the latex particles or acting as coalescing aids themselves. There was a larger slope change for Efka PL 5651, which indicates a difference in interaction between the two coalescing aids and the latex.
Upon these results, it was concluded that with differing technology (i.e., different acrylics and styrene-acrylic) tested, performance of coalescing aids should be evaluated for individual systems. Historically, Texanol has been found to be an excellent coalescing agent across multiple systems. With ultra-low VOC coalescents that remain in the films, efficiency is certainly an important consideration. Formulators drive to reduce the amount of low molecular weight materials, which at higher loadings can lead to more poor paint performance properties.
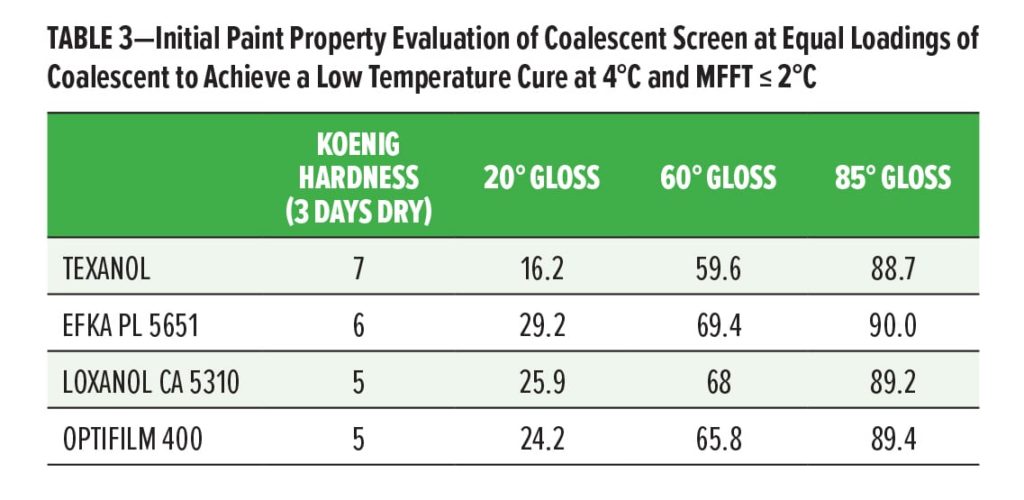
To evaluate the effects on paint properties, the three studied ultra-low VOC coalescing agents as well as a traditional volatile coalescent, Texanol, were compared in a 50 g/L VOC interior, semi-gloss paint formulation. Equal loadings of coalescent were used on resin solids and the resulting paints were measured for multiple properties. The properties considered most critical when evaluating between volatile and nonvolatile coalescents are reported herein. Gloss, stain resistance, hardness, and scrub resistance were evaluated. Scrub resistance of the particular formulation was not found to be affected by the zero-VOC coalescents, and all values were greater than 2400 cycles.
One interesting finding was the effect of the ultra-low VOC coalescents on gloss potential compared to the volatile Texanol. Displayed in Table 3, all permanent coalescent systems showed gloss values between 65–70 units at 60° inference, compared to a 59.6 for the volatile coalescent system. As film formation improves, gloss typically rises due to the smoothness of the coherent film formed. In this case, Texanol may be too volatile prior to the paint drying to achieve maximal gloss.
Hardness, which is often a concern with permanent coalescents, was found to drop in comparison to the volatile Texanol-based system. Both the Loxanol CA 5310 and Optifilm 400, which were found to be less efficient than the Efka PL 5651, showed lower pendulum hardness even though the loadings of the coalescing aids were the same (Table 3).
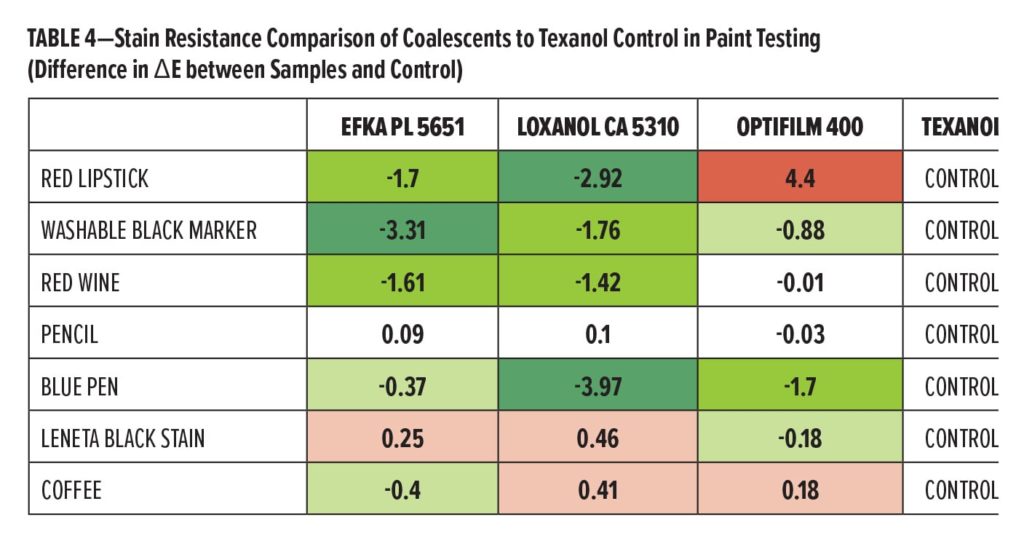
The most surprising effect found in this particular paint system was in the stain resistance evaluation. Using the Texanol-based paint as a control, ΔE values were measured for each stained area compared to unstained panel to indicate strength of stain removal. ΔE values were then measured for the individual stains in the three permanent coalescent systems. ΔE values for a given stain were compared to the ΔE value for that same stain measured for the Texanol control. In Table 4, the difference in the ΔE values is reported for each stain in each system compared to the measurement in the Texanol control paint. Therefore, the more negative the number, the more improved removal of the stain was seen compared to the control. Likewise, a positive number reported indicated an increase in ΔE for a given stain compared to the control, indicating worse staining after cleaning. This is also represented by a colored heat map where green is improved stain removal compared to the control, and red indicated worse stain removal compared to the control. The resulting comparison indicates that ultra-low VOC coalescents, dependent on structure, may assist in improving stain resistance. Efka PL 5651 was found to have marked improvement in washable marker, red wine, and lipstick stains. Loxanol CA 5310 showed improvement in the lipstick, black marker, red wine, and blue pen. Optifilm 400 varied slightly from the Texanol control, with lipstick actual showing less stain removability. The improvement in both aqueous hydrophilic and hydrophobic stain was unexpected (Figure 6).
Conclusions
Through comparison of ultra-low VOC coalescing aids, the necessity for formulators to evaluate coalescents independently for each system was found to be critical. The efficiency differences between coalescents Optifilm 400, Loxanol CA 5310, and Efka PL 5651 were found to be significantly dependent on the system in which it was being investigated. Stain resistance was also found to be improved compared to Texanol and the other coalescents evaluated in this study. Less hydrogen bonding character may play a more substantial role in improving stain resistance with respect to the coalescent.
® registered trademarks of BASF Group. © 2017 BASF Corporation
Disclaimer
While the descriptions, designs, data, and information contained herein are presented in good faith and believed to be accurate, they are provided for guidance only. Because many factors may affect processing or application/use, BASF recommends that the reader make tests to determine the suitability of a product for a particular purpose prior to use. NO WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, ARE MADE REGARDING PRODUCTS DESCRIBED OR DESIGNS, DATA OR INFORMATION SET FORTH, OR THAT THE PRODUCTS, DESCRIPTIONS, DESIGNS, DATA OR INFORMATION MAY BE USED WITHOUT INFRINGING THE INTELLECTUAL PROPERTY RIGHTS OF OTHERS. In no case shall the descriptions, information, data, or designs provided be considered a part of BASF’s terms and conditions of sale. Further, the descriptions, designs, data, and information furnished by BASF hereunder are given gratis, and BASF assumes no obligation or liability for the descriptions, designs, data, or information given or results obtained all such being given and accepted at the reader’s risk.
This paper was presented at the 44th International Waterborne, High-Solids and Powder Coatings Symposium, February 22-24, 2017, in New Orleans, LA.
CoatingsTech | Vol. 14, No. 4 | April 2017