By Celine Burel, Wendy Lu, Madi Ngene, Remi Giordanengo, Romane Tranchard, Yuyi Liao, and Lichang Zhou, Syensqo
The water resistance of waterborne all-acrylic and styrene acrylic dispersions is investigated at different Tgs. The performance of films obtained from latexes stabilized using conventional surfactants (SLS and Surf 1) is compared to that of latexes stabilized using polymerizable surfactants (PolySurf 1 and 2). The effect of the surfactant concentration and surfactant chemistry on the water whitening and water uptake of the latex films is studied. The surfactant chemistry and concentration, as well as the pH of the final latex have a significant influence on the water resistance results. The water whitening phenomenon is directly linked to the latex colloidal stability during drying and the excess of surfactant in the continuous water phase. Atomic Force Microscopy (AFM) images revealed that labile surfactants can migrate within the film during drying and form hydrophilic pathways or pockets, making the film more susceptible to water at higher concentration of surfactants. Proton nuclear magnetic resonance (1H-NMR) analyses, combined with AFM imaging, showed that when polymerizable surfactant is used, due to its incorporation into the polymer backbone, the residual amount of surfactant in the continuous phase is low, resulting in films with superior water resistance. Moreover, the controlled distribution of the polymerizable surfactant on the latex particles results in a more homogeneous distribution of the surfactant in the latex film lowering the water sensitivity of the films. Overall, for Tgs between -5 °C and 10 °C in acrylic latexes, the PolySurf2 exhibits superior water whitening and water intake performances as compared to conventional surfactants. PolySurf2 is designed to provide superior water resistance performances in clearcoats.
Introduction
Clearcoats are widely used in coatings markets such as industrial and architectural paints as an invisible top layer to protect the underlying paint coats from UV rays, dirt, and water. Indeed, paints in direct contact with rain or a high-humidity environment require extra protection against water penetration. Because of additional environmental regulations and recent market trends toward more sustainable technologies, solvent-based formulations that provide excellent water resistance are under scrutiny, and waterborne clearcoats have become more desirable.
Waterborne polymers used in clearcoats are environmentally friendly, easy to handle, relatively low in cost, and can be made with high-solids content through emulsion polymerization. Although surfactants and hydrophilic monomers are necessary during the latex synthesis to provide steric and/or electrostatic stabilization to the particles formed, their distribution during drying can impede the water resistance of the polymer films. The phenomenon of water whitening in polymer films has been mainly attributed to water molecules “clustering” near the polar constituents of the films, such as the surfactants and the hydrophilic monomers.1-4 Moreover, during the film formation, surfactants can migrate toward interfaces, forming hydrophilic pockets within the polymer film or concentrate near the surface of the particles to create hydrophilic pathways throughout the film.1-5 The water-swollen pockets scatter light and make the film appear cloudy or white.6,7 To reduce the negative effects of conventional ionic surfactants on the water sensitivity of the latex films, polymerizable surfactants are used. These reactive surfactants polymerize with the monomers and are incorporated into the backbone of the polymer chains of the latex.
Polymerizable surfactants are then locked on the latex particles, and a better control of their distribution within the polymer film is achieved during drying because of the lack of both desorption from the latex particles and migration in the polymer film.8-10 Although the mechanisms of water sensitivity of polymer films have been extensively studied before, to the best of our knowledge, a broad analysis of the water whitening and the water uptake of clear latex films as a function of Tg has not been reported to this date. The effect of the latex pH on the water whitening resistance of the final films was also studied. In addition, the effect of surfactant concentration on the latex films’ water-resistance properties was investigated. Finally, it was demonstrated that the polymerizable surfactants used in this study are almost fully converted during latex syntheses leaving very little unreacted surfactant in the dried polymer films, which enhances the water-resistance properties of thus films.
Materials and Methods
Materials
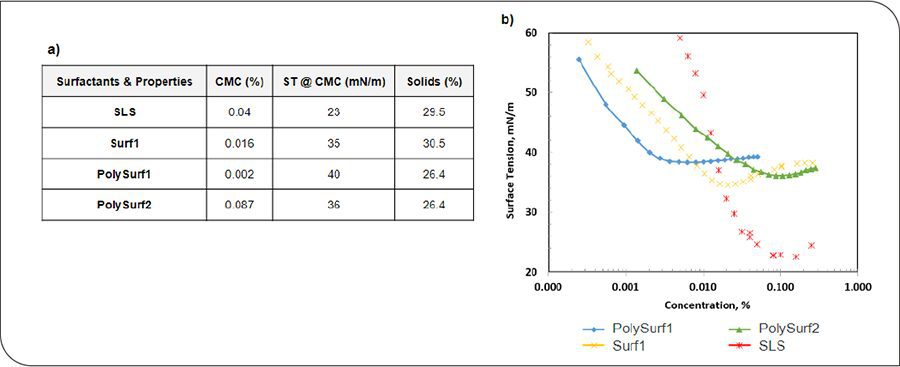
Methyl methacrylate (MMA), butyl acrylate (BA), and methacrylic acid (MAA) (99%, Sigma-Aldrich) were used as is. Surf1 (Syensqo), SLS (Syensqo), PolySurf1 (Syensqo), and PolySurf2 (Syensqo) were used as emulsifiers in the latex syntheses. Sodium bicarbonate (99.7%, Sigma-Aldrich) was used as a buffer in the preparation of the latex surfactant control. Ammonium persulfate (99.8%, Sigma) was used as an initiator in all latex synthesis. Deionized (DI) water was used throughout the work. Figure 1 presents the main characteristics of the surfactants used throughout this study.
FIGURE 1 (a) Surfactants’ physico-chemical properties; (b) concentrations.
Continue reading in the March-April digital issue of CoatingsTech
