By Leo J. Procopio, Paintology Coatings Research LLC
Paints and coatings are typically used to beautify and protect, but there are many examples of specialty coatings that serve other functions.1,2 The development of these “functional” coatings has been a trend in the industry for many years, and there are numerous examples such as soft-feel coatings for consumer electronics3,4, sound-damping coatings for mitigating noise in automobiles5,6, and antimicrobial coatings designed to kill microorganisms that come into contact with the coated surface7. Another trend in the paint and coatings industry has been the development of coatings that control the use of energy.
Access to energy is an important global driver for economic growth, and how we generate, efficiently use, and ultimately conserve energy has important consequences for the future of our environment and society. Coatings technology has an important role to play in this ongoing struggle.8 For example, coatings that can be cured at lower temperatures inherently use energy more efficiently.
The replacement of heavier bitumen pads with lightweight liquid-applied sound-damping coatings allows auto manufacturers to remove weight from automobiles.5,6 Reducing weight of transportation vehicles uses energy more efficiently and improves mileage. Antifouling coatings help the fuel efficiency of ships by preventing the buildup of biofouling on the hull, which increases drag and makes engines work harder to achieve the same result.9,10
Several types of functional coatings are targeted at managing thermal energy. Cool-roof coatings keep the interior of buildings cooler and lighten the load on air conditioning during the hot, sunny days of summer. High solar reflectivity and thermal emissivity helps the coating deflect energy in sunlight, preventing the roof from heating up as much, and thus less heat is conducted through the roof and into the building.11,12 Cool coatings for exterior building walls also function in a similar manner.
Cool coatings also help defend against the urban heat island effect, where urban environments with large areas of dark roofs and paved surfaces tend to be warmer than nearby rural areas. Thermal insulation coatings are also used to manage thermal energy for both personnel protection and energy conservation purposes.13 However, thermal insulation coatings rely on a different mechanism and prevent heat transfer between materials due to their low thermal conductivity.
In this article, we introduce thermal insulation coatings and the science behind how they work. First, a discussion on the physics of heat transfer and thermal conduction will provide some necessary context to understand how insulation works. A description of traditional insulation materials and some lingering problems with those materials will give perspective into why thermal insulation coatings were developed, followed by a description of how thermal insulation coatings are formulated, applied and perform. A brief comparison with cool-roof coatings will also be given to clarify common misunderstandings about functional coatings and how they each help with energy management.
MECHANISMS OF HEAT TRANSFER
The flow of heat between materials is controlled by three basic mechanisms: conduction, convection, and radiation. Consider the simple scenario of heating water in a pot shown in Figure 1, which is often used to explain the three mechanisms. When heat flows through a solid material, it is by conduction. An example of conduction is the flow of heat from the fire, through the metal of the pot, to the hand holding the pot handle. The rate of conductive heat transfer depends on the chemical nature and structure of the solid material. If the pot in Figure 1 is a cast iron skillet, the cast iron handle could get very hot, and an oven mitt might be needed to touch the handle with your hand. Many pots have handles made with or covered by a different material such as wood or plastic. The conduction of heat through those materials is slower than through metal, so pots with those handles can often be held with a bare hand.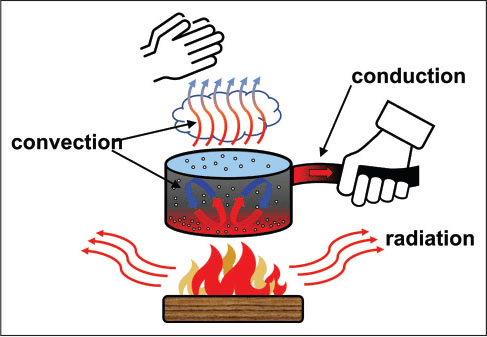
Convection is the transfer of heat by the movement of a fluid; either a gas or liquid. In Figure 1, heated water moves from the bottom of the pot, which is closer to the heat source, upward toward the cooler surface. In this case, convection involves the movement of a liquid. In a similar manner, convection involving the movement of a gas is a process that causes warm, lighter air to rise and cold, denser air to sink within a house, resulting in upper floors often being warmer than the lower floors. Another example of convection involving a gas is shown in Figure 1, where the boiling water evaporates as steam, which rises from the hot water surface and heats up the cooler air above. In these examples, convection occurs because of differences in the density and buoyancy of hot and cold regions of the liquid or gas. Hotter, less dense fluids will tend to rise, and colder, denser fluids will descend.
Heat can also be emitted from a material through radiation in the form of electromagnetic waves, such as infrared (IR) radiation. Heat transfer through radiation results in the warmth we feel when we hold our hands near a fire, as shown in Figure 1, or when sunlight heats a dark roof or an asphalt parking lot. Anyone walking in bare feet on hot asphalt pavement during a sunny summer day experiences the result of heat transfer by radiation. Radiation is also the mechanism through which the hot roof or asphalt pavement releases (or emits) heat into the surrounding air and cools down once the sun sets.
