By C. Jim Reader and Maria Nargiello, Evonik Corporation
The field of coatings technology has utilized many forms of silica-based particles in the last 70 years. This large, varied class of fillers is generically broken into two categories of crystalline and amorphous morphology. With ongoing scrutiny and sensitivity in the coatings industry to move towards less hazards in the workplace, greater emphasis is placed on suitable amorphous technology to replace crystalline silica technology. Amorphous silica is highly adaptable and flexible to be modified in both powder and pre-dispersed forms, and numerous engineered types of technologies have been developed to provide functional solutions to many coatings problems.
Amorphous silica technology has been developed to address functionalities including: rheological control, suspension of pigments and fillers, and reinforcement of coatings film; to impart scratch resistance, hydrophobicity / anti-corrosion benefits, and oleophobicity; as a carrier of trace actives into coatings for homogenous distribution; for flow control, charge, and fluidization enhancement of powdered coatings; and gloss reduction of liquid systems. Particle technology and modification will be addressed along with performance attributes highlighted for each of the types of tailor-made modifications. The importance of proper dispersion and homogenous distribution within a coating matrix will be reviewed.
This article will address how amorphous silica technology is differentiated and engineered to create specially tailored solutions to enhance the performance of coatings and will highlight the latest technical developments in this field.
Introduction
Silica, or silicon dioxide, is one of the most abundant minerals present on earth. It is estimated that quartz, the most stable form of this complex family of materials, makes up more than 10% of the earth’s crust and, as a major component of the natural sands widely used in the construction industry, is a key raw material to produce glass and silicon.1
Silica is also an important raw material for the coatings industry, as it can provide a wide range of functionalities and benefits. These include rheological control, enhanced film formation, improved mechanical properties of the final coating film, free flow and fluidization enhancement of powders, and control of gloss. Silica is also an important raw material for the formulation and production of defoamers. The silica grades used in the coatings industry are produced synthetically and typically meet greater quality control standards, often having tighter physical-chemical requirements, such as color and brightness. The enormous variety of performance properties is achieved by adjusting the particle size and morphology during production as well as via surface treatment and densification of the silica particles in downstream processes.
A summary of the main methods for producing synthetic silica is shown in Figure 1. The most common types of silica used in modern coatings are produced either by a liquid phase process of precipitation or gas phase process of flame hydrolysis. Precipitated silica is produced by the controlled reaction of sodium silicate (“water glass”) and sulfuric acid similar to the production of silica gels. The silica is precipitated, filtered, washed, and dried before milling and classification (Figure 1).
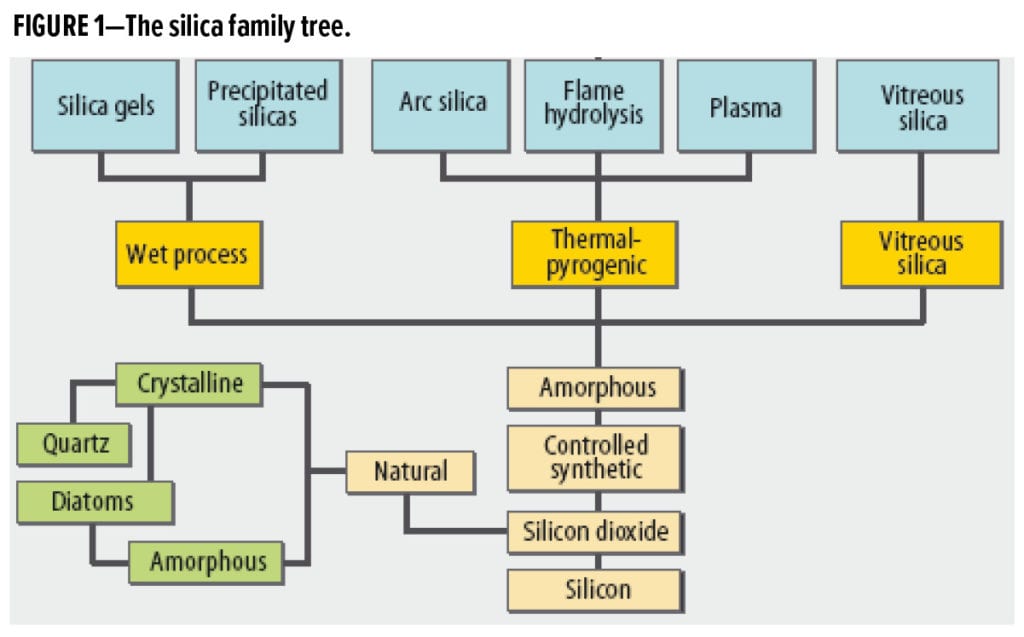
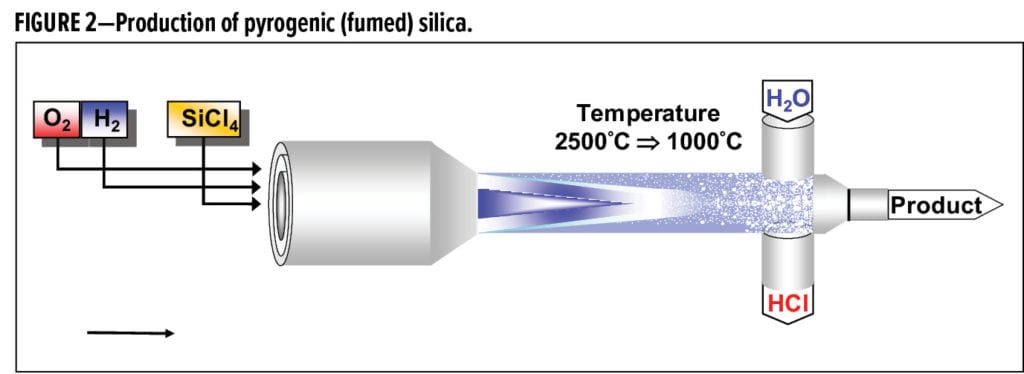
The production of fumed silica began with the discovery of the flame hydrolysis of silicon tetrachloride by Harry Klöpfer in 1943. This discovery was part of a wartime effort to produce silica that could act as a white reinforcing filler to modify rubber, which was then much needed for tire production to replace oil used to make carbon black. A simple diagram of the process is shown in Figure 2. The overall chemistry of the process is efficient and versatile. A vaporizable metal precursor is fed into a hydrogen/air flame, and the hydrolysis product, silicic acid for instance, rapidly condenses to the metal oxide. Multiple pathways to particle formation are possible, such as particle growth through deposition, particle evaporation, aggregation, and aggregate coagulation. The elegant efficiency of the overall chemistry makes the process very amenable to variation. A diverse array of metal oxides beyond silica has been produced, including mixed metal systems and surface modified and doped particles that can be used for a wide variety of industries and applications.
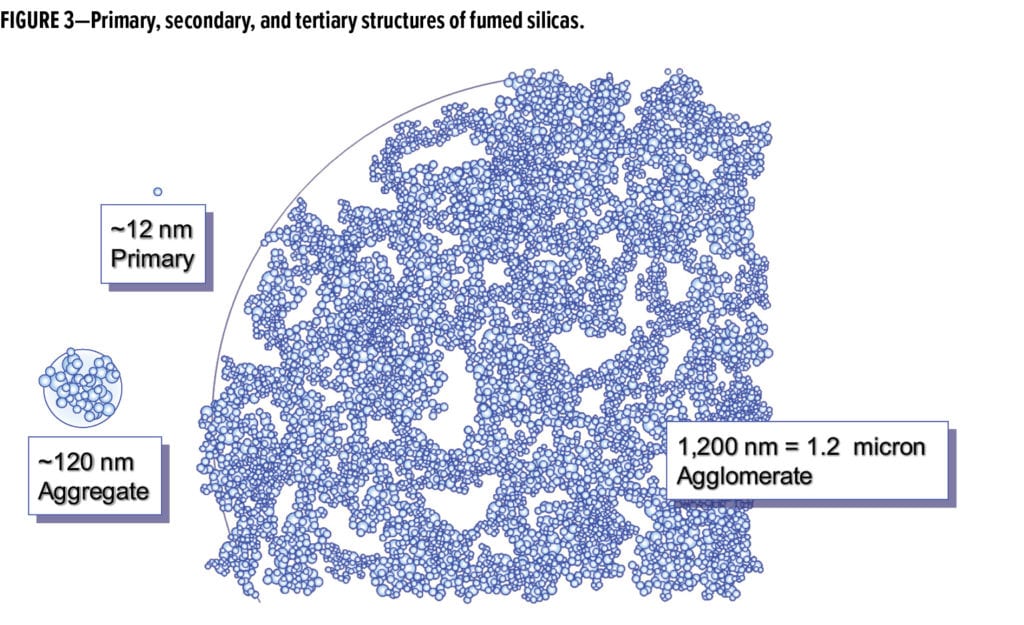
Fumed silica consists of three conceptual levels of structure (Figure 3). The primary particle only exists for a short time in the flame. Primary particles fuse together to form an aggregate, which is the secondary particle structure. Isolated primary particles, in this model, are rare. The tertiary structure is an agglomeration of the secondary structures. This collection of particle aggregates can be disrupted by the introduction of shear, and then reform over time after the shear is removed from the system. This mechanism is the means by which fumed silica imparts pseudo-plastic rheological properties to formulations.
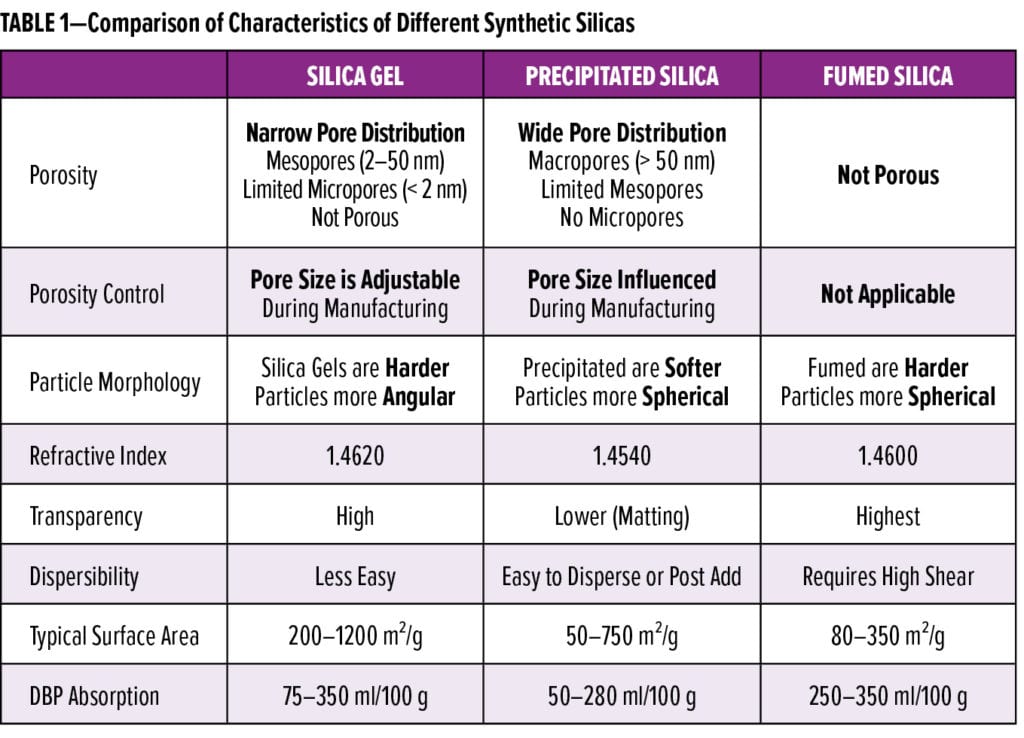
A comparison of the different physical properties of synthetic silica is shown in Table 1. It is important to note that all of these synthetic silica types are amorphous and do not contain crystalline silica. This has been confirmed by X-ray diffraction.
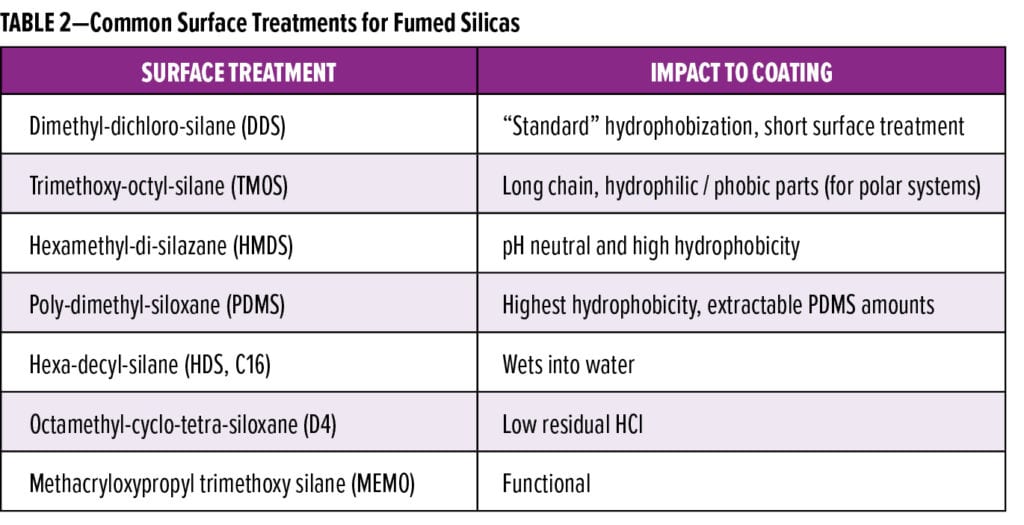
The second element of particle design is surface modification to render the hydrophilic particles hydrophobic in character. This is achieved by the reaction of the surface silanol groups with different silanes. These treatments create different grades of fumed silicas that vary in hydrophobicity, tribo-electrostatic charge, and thickening efficiency. A summary of the typical surface treatments with corresponding attributes is shown in Table 2. The level of treatment, which can be measured by carbon content and methanol wettability (Figure 4), indicates the consistency of treatment and the balance of hydrophilic to hydrophobic surface.
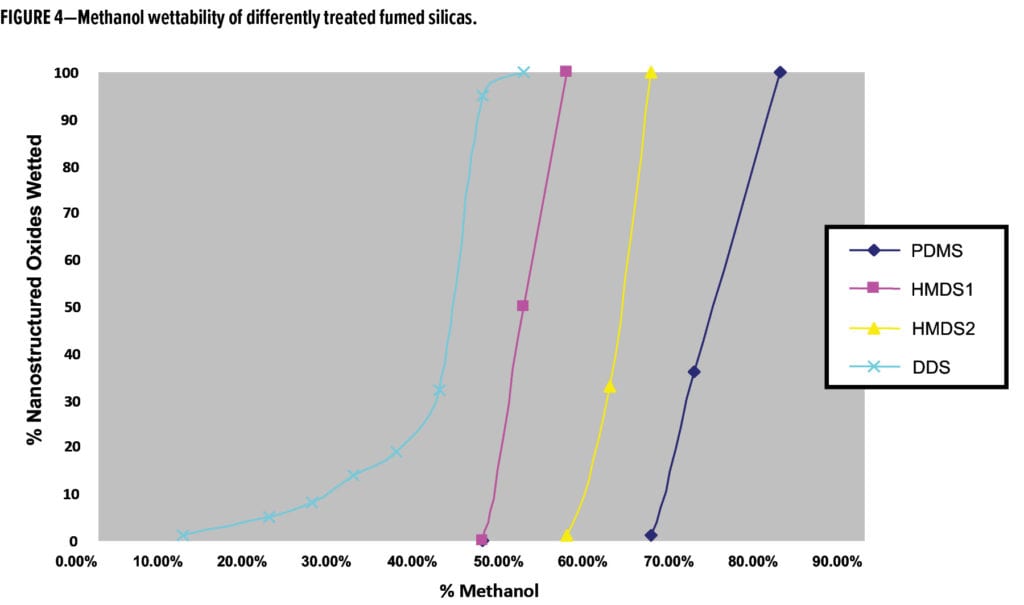
The Multipoint Methanol Wettability method is a quantitative test method to measure the level and consistency of hydrophobic treatment. The 0.2 g of the treated silica is added to a series of graduated test tubes containing 8 ml of dilutions of methanol in water made in 5% increments, starting with 100% water, 95% water, and 5% methanol up to 100% methanol. The silica/solutions mixtures are shaken and then centrifuged under controlled and defined conditions. Depending on the level of hydrophobicity and consistency of surface treatment, the silica will wet differently into each water. Methanol mixtures and the amount of wetted silica in each solution mixture is recorded and plotted to a curve known as the methanol wettability fingerprint. Silicas requiring higher methanol amounts for wetting are more hydrophobic. Consistently treated silica shows a steep rise in wet-in, whereas a more gradual curve indicates a wider range in the consistency of treatment. Precipitated silicas can also be surface treated, typically with waxes and reactive oligomers, to improve product and formulation stability and reduce viscosity impact.
The third element of particle design is structure modification via one of several proprietary processes. Granulation results in larger, individual spherical particles in the range of 20–30 μm that are porous; their main function is to act as free-flowing carriers of liquid-based actives and oils. Other chemical and mechanical post-processes reduce structure (i.e., the level of aggregation or agglomeration). Products resulting from post-processing can have significantly higher bulk densities and dramatically reduced thickening efficiency due to reduced levels of aggregation at the primary aggregate level. The functional benefit resulting from such grades are enhanced scratch and abrasion resistance, as higher loading can be achieved with minimal impact to formulation viscosity. This higher loading results in reinforced domains, which drives the scratch and abrasion resistance.
Rheology and Film Formation
Fumed silica, in various grades and modifications, has been used for decades in coating formulations to impart thixotropy, anti-settling, and anti-sag properties. The main requirements for good performance are proper selection and adequate dispersion to homogenously distribute aggregates throughout the coating matrix. Proper grade selection can be loosely correlated to dosage, particle size, structure, and surface treatment. Untreated, hydrophilic fumed silica grades give the best performance in non-polar environments, whereas hydrophobically modified grades, such as those treated with DDS, TMOS, and HMDS (Table 2) are more efficient as polarity increases. This trend is shown in Figure 5.
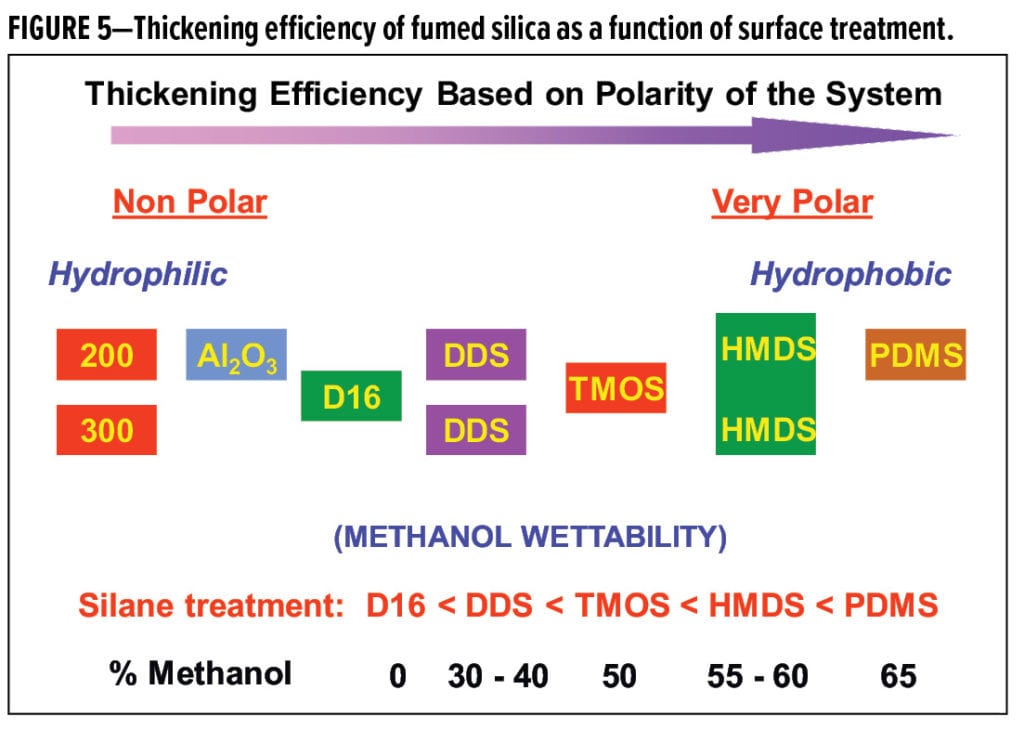
Grades treated with TMOS and HMDS are highly effective for high solids and radiation-cure systems. Polydimethylsiloxane-treated grades are the most hydrophobic. This technology can also be considered in high solids and 100% solids systems, where it is very effective. However, care must be taken, as this surface modification is not fully reacted to the surface, and migration of the free PDMS may cause surface defects or adhesion problems.
Proper dispersion of the fumed silica is critical to good performance. When optimizing a dispersion for thickening efficiency and rheological enhancement, several parameters should be considered including shear rate. Dispersion time, temperature control, and sequence of addition are all important. High-speed dispersion using a saw-type blade at a shear rate > 10 m/s is recommended. Longer dispersion time will not compensate for inadequate shear rate. The consequences of poor dispersion are typically larger agglomerates that remain visible in the final coating, reduced thickening efficiency, poor thixotropic stability over time, lower gloss and transparency, and possibly film defects.
Fumed silica has excellent thermal stability, but as the temperature of the coating environment increases, particularly upon shear, the wetting properties of the coating typically improve. This can lead to over-dispersion, whereby the aggregates are reduced past their optimum association level. Once this occurs, reduced thickening efficiency results, sometimes to the point where it appears as if no thickener was added. Fumed silica is one of the smallest particle size materials added to a coating formulation. This component should be added early in the formulation, preferably to the resin or binder, rather than into non-film-forming components like solvents for optimal effect. Caution should be used when post-adjusting batches with powder, as only minimal shear that is inadequate for homogeneous incorporation can often be used at this stage. Post addition with low shear may bring formulations to their desired rheology, but this can deteriorate over time, as larger agglomerates slowly wet-out.
Lab evaluations have demonstrated that multiple performance attributes can also be enhanced using fumed silica dispersions. These include improved suspension of pigments, fillers and matting agents, reduced tack, improved dirt pick-up resistance, enhanced film strength, and even improved film formation. These features are achieved without compromising gloss and other appearance attributes. An example is shown in Figure 6, where a pre-made aqueous fumed silica dispersion improves the film formation in combination with reduced coalescent solvent levels. These improvements have been seen with many different film-forming resins and using less or no coalescing solvents. This can help reduce the overall volatile organic compounds of the formulation, in addition to providing the other properties referenced above. This enhanced film formation is a result of reduced stress propagation due to the reinforcing effects of the very finely dispersed fumed silica.9

Anti-Corrosion/Water Repellency
Hydrophobically modified grades of fumed silica have also been used together with anticorrosive pigments to improve corrosion performance and water repellency of coatings. These grades are not considered to be anti-corrosion pigments, but they work effectively with many classes of anti-corrosive pigments such as modified barium metaborate, calcium phosphosilicate, and zinc dust. Loadings between 1.0% and 3.0% by weight of total formulation are used to ensure that there are enough particles in the coating matrix to support a hydrophobic barrier, improve the mechanical properties of the film, and increase hydrophobicity. Water repellency measured by improved blister resistance can be improved at lower loading levels starting at 0.5% by total formulation weight. Two examples of this effect are shown in Figure 7.

Proper dispersion is again needed to homogeneously distribute the silica throughout the coating matrix for maximum effect. It is suggested that the treated fumed silica be dispersed together with the anti-corrosion pigments to ensure optimal dispersion. The best results have been obtained using DDS, TMOS, and HMDS treatments, although the impact on formulation rheology must also be considered.
Scratch Resistance
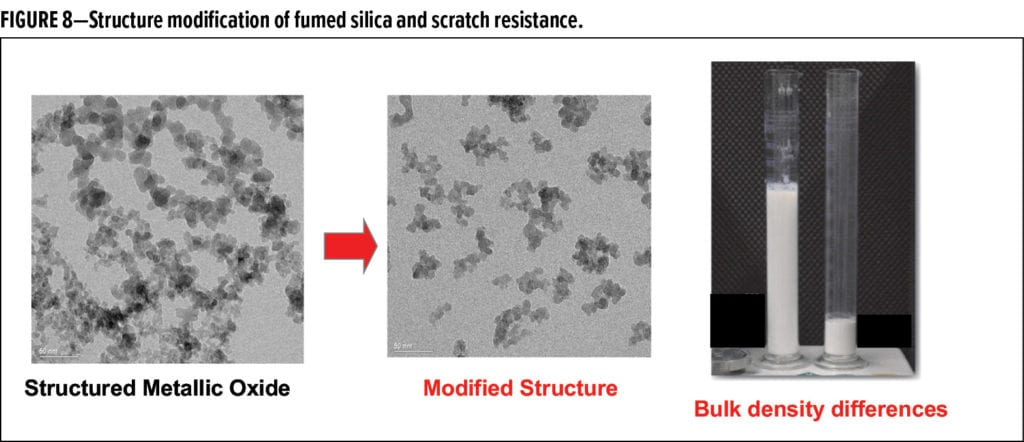
The development of silica particles for the specific purpose of improving scratch resistance came from the creation of particles for high reinforcement of elastomers and composites. The critical factor needed to achieve high reinforcement is to be able to fill the polymer matrices with significantly higher levels of fumed silica without increasing the viscosity to unworkable levels. This is achieved by structure modification through a proprietary post-processing to achieve a highly reduced structure and a low level of aggregation. This results in a material that can be used as a reinforcing filler to increase the mechanical strength of the coating and impart scratch resistance. The corresponding physical property observed is a significant increase in the bulk density (Figure 8) and a dramatic reduction in the ability of silica to increase viscosity. When compared to other reinforcing fillers, such as alumina, silica has the advantage of a lower refractive index of 1.46, more closely aligned with many polymer systems, which results in improved transparency and clarity. These materials can also be hydrophobically modified with DDS, HMDS, and TMOS for improved water resistance.
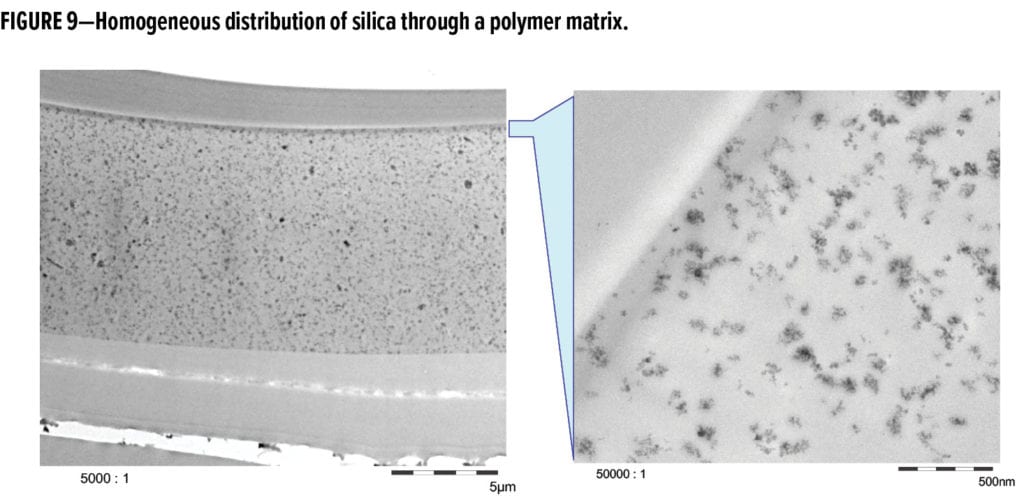
The main consideration for successful use of surface-treated, structure-modified fumed silica particles is adequate loading level. Optimum loading levels start at 5% by weight on total formulation and can approach 15%. Inorganic particle load must be high enough to attain a homogenous density through the polymer to achieve a consistent, reinforced matrix. This can be seen in the scanning electron microscopy (SEM) analysis of a cross-section of a high-solids coating (Figure 9), which shows a homogenous distribution through the film with no surface enrichment resulting from higher particle density.
Five percent loading of an easy-to-disperse (E2D) structured modified silica particle treated with DDS achieved improved scratch resistance in a high-solids system, tested by a dry scratch method using a Crock meter (abrasive paper) and wet scratching using an Elcometer (40 double strokes, bristle brush, and 0.15% quartz in water slurry). Improved scratch resistance and higher gloss retention of the coating was observed after using both scratching methods, and reduced haze was observed after the panels were subjected to Elcometer testing (Figure 10). The addition of 5% silica did slightly reduce gloss, but the silica significantly improved scratch resistance.
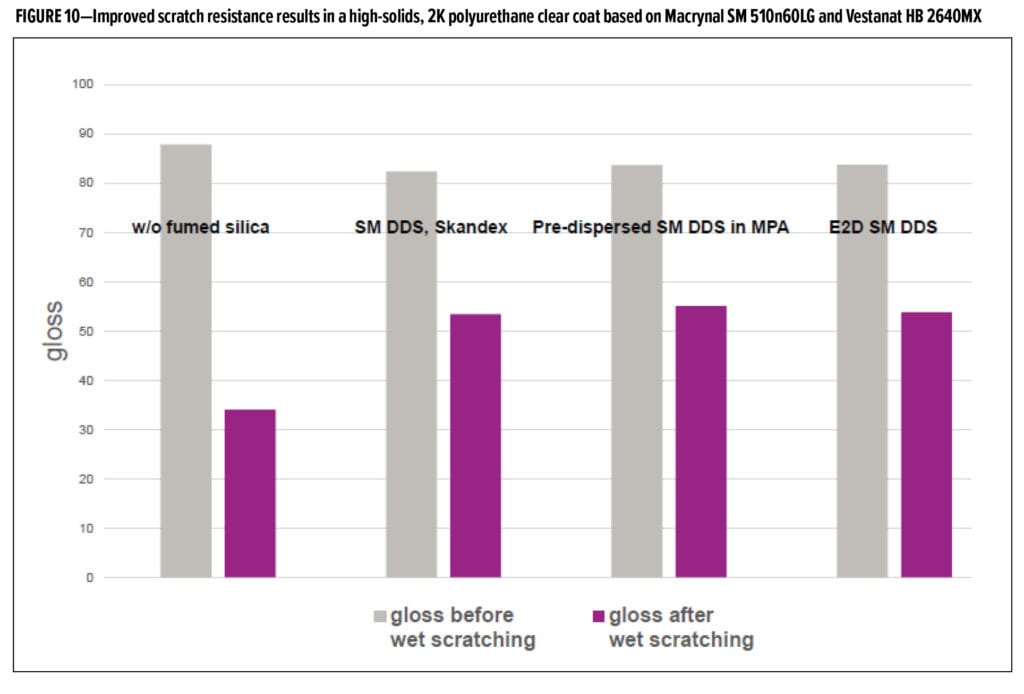
Results in Figure 10 show three variants of DDS-treated structure-modified silica. Variant 1 is the original that requires milling, variant 2 the same DDS structure modified silica pre-dispersed in methoxypropyl acetate (MPA), and variant 3 is the newest version, which is easy-to-disperse.
Free Flow, Fluidization, Transfer Efficiency
Powder coatings, whether conventional, fine, thermosetting, thermoplastic, tribo, or UV-cured, all require good flow, reduced moisture pick-up, good package stability (no caking), efficient fluidization, and high-transfer efficiency as well as reduced Faraday cage effects for even film thickness and optimized appearance during application. Hydrophilic- and hydrophobic-treated fumed silica and alumina can be used to improve flow, storage stability with reduced moisture pick-up, and improved fluidization and transfer efficiency.
In practice, flow additives used in powder coatings can be added in one of three places during the powder coating manufacturing process: 1) directly in the hopper, 2) dosed into the powder during chipping, or 3) post-added after pulverization. Flow additives can be dry blended into problematic powdered components before they are charged into the hopper to help them feed more consistently and homogeneously into the extruder. The typical loading of flow additives used in this step is 0.1–0.3%. Flow additives used to pre-treat ingredients are extruded into the powder matrix and do not influence the bulk flow properties after compounding and pulverization.
When additives are used to influence the final powder coating properties, they must be added after extrusion and be oriented on the outside of the powder coating particles. There are typically two places where silica or alumina (or a combination of the two) can be added into the process to achieve this: 1) prior to chipping where the additive is cut into the powder coating particle or 2) after pulverization and classification. Care should be taken when dosing the additive before pulverization, as classification systems can remove the additive out of the powder and reduce the final dose remaining in the powder coating. The typical dosage level used in the chipping or as post add is also 0.1–0.3% by weight.
A study was organized with the University of Western Ontario to assess specific performance attributes associated with four classes of additives in different powder coatings. The first two powder coatings were corona applied. The first was a conventional polyester with d50 of 31.5 μm and the second was a finer particle size, polyester with d50 of 21.5 μm. The additive dosage level was adjusted based on the particle size of the powder. A 0.3% dosage level was used for the coarse powder and a 0.5% dosage level for the fine powder. A third powder was a tribo-applied polyester powder coating. The attributes tested were angle of repose (flowability), bed expansion (flowability and fluidity), transfer efficiency, Faraday cage effects, gloss, and gel time. Four types of silica were tested: untreated hydrophilic silica with a surface area of 200 m2/g, HDMS- and aminosilane-treated silica with a surface area of 200 m2/g, DDS-treated silica with a surface area of 130 m2/g, and HDMS-treated silica with a surface area of 300 m2/g.
Surface-treated alumina was most effective at improving transfer efficiency and reducing Faraday cage effects due to its neutral to slightly positive electrostatic charge character. This is shown in Figure 11 where a disk applied with coarse powder containing 0.3% high surface area alumina (130 m2/g) has a more consistent level of jetness than the disk applied with powder containing no additive. This test measures how much powder is transferred and the consistency of coverage of the disk (by weight) under controlled application conditions.
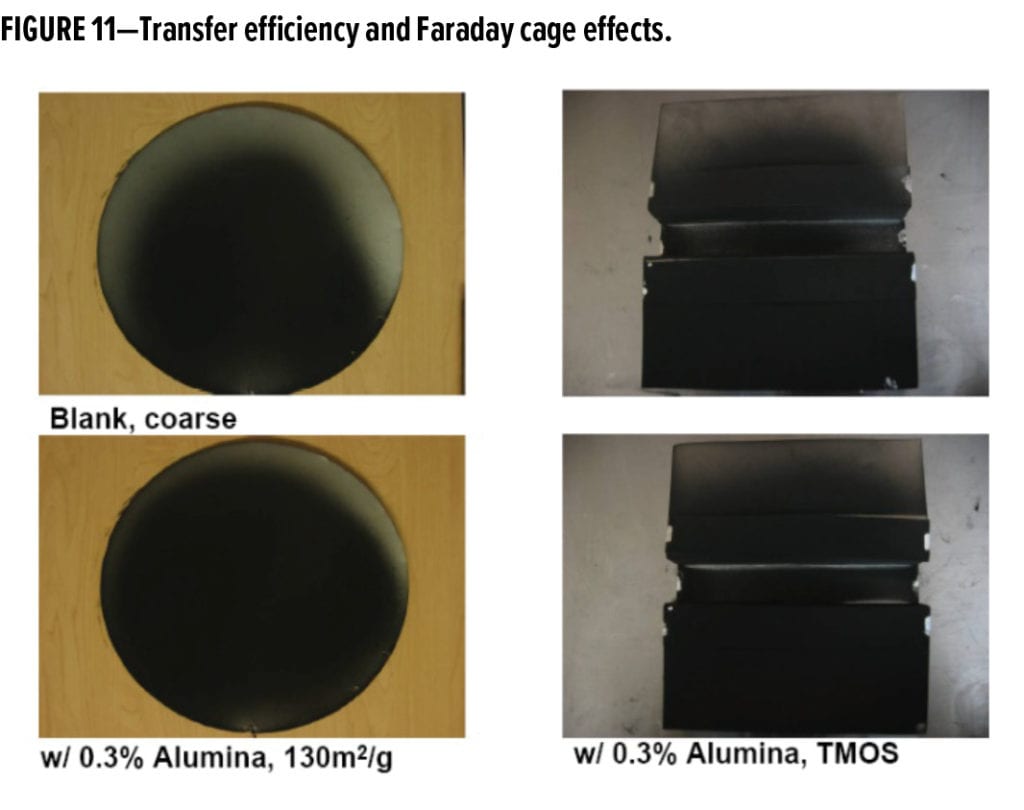
Faraday cage effects were measured by determining how much coating is deposited in the inner trough of a test specimen. The interior parts of the trough have three removable panels under controlled applications conditions. After application, these inner panels are removed and weighed. Reduced Faraday cage effects (improvement) are associated with higher, more consistent weights of powder deposited on these inner removable panels. The 0.3% alumina treated with TMOS was effective in reducing Faraday cage effects in the coarse, black powder coating.
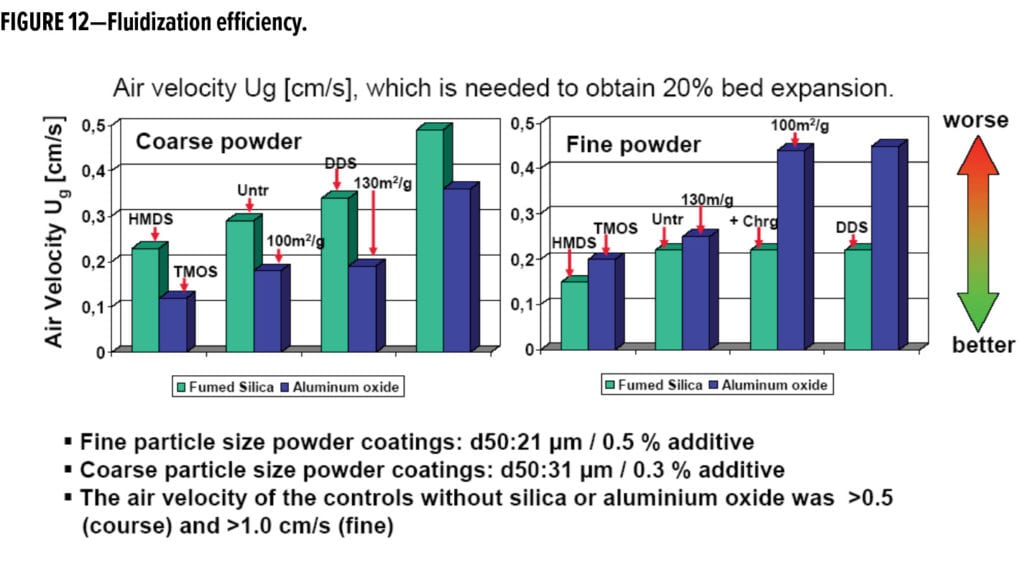
Fluidization efficiency was also assessed in this study. The results in coarse and fine powder show that particle size of the powder coating significantly affects the type of additive most effective for improving fluidization. Alumina was more effective in improving fluidization in the coarse powder coating as measured by lower air velocities needed to obtain 20% bed expansion, while silica was more effective in the fine powder coating (Figure 12). This trend suggests that additive packages may need to be adjusted based on their particle sizes.
Gloss Control
Gloss is defined, according to DIN EN ISO 4618, as the human perception of the more-or-less directed reflection of light rays from a surface. Glossy surfaces appear shiny and reflect most light in the specular (mirror-like) direction, while matte surfaces diffuse most of the light in a range of angles. Gloss level can be characterized by the angular distribution of light scattered from a surface, measured with a glossmeter or reflectometer, and it is dependent upon the viewing angle (Figure 13).
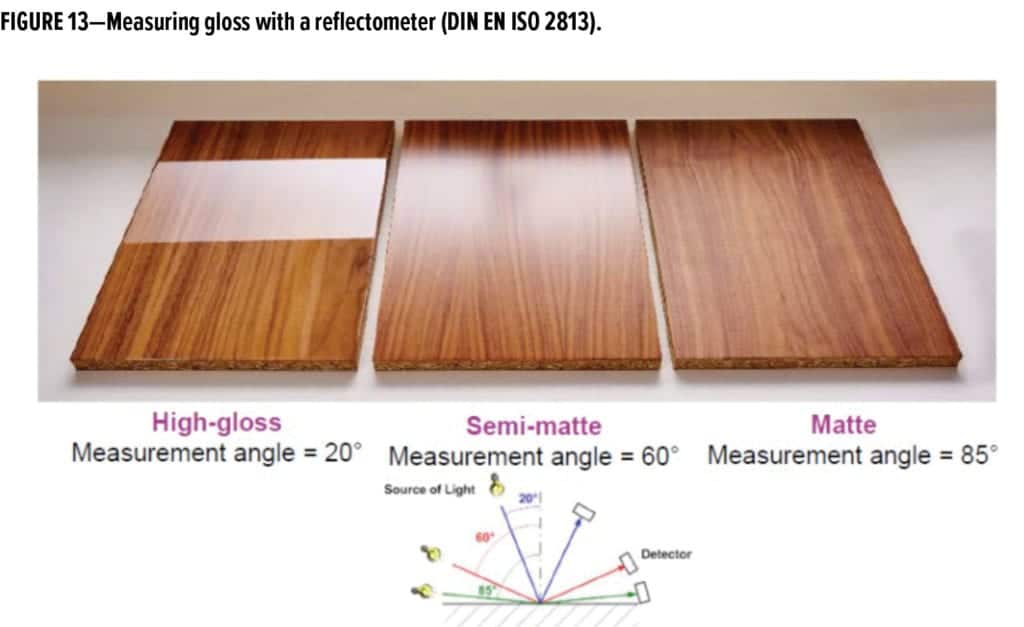
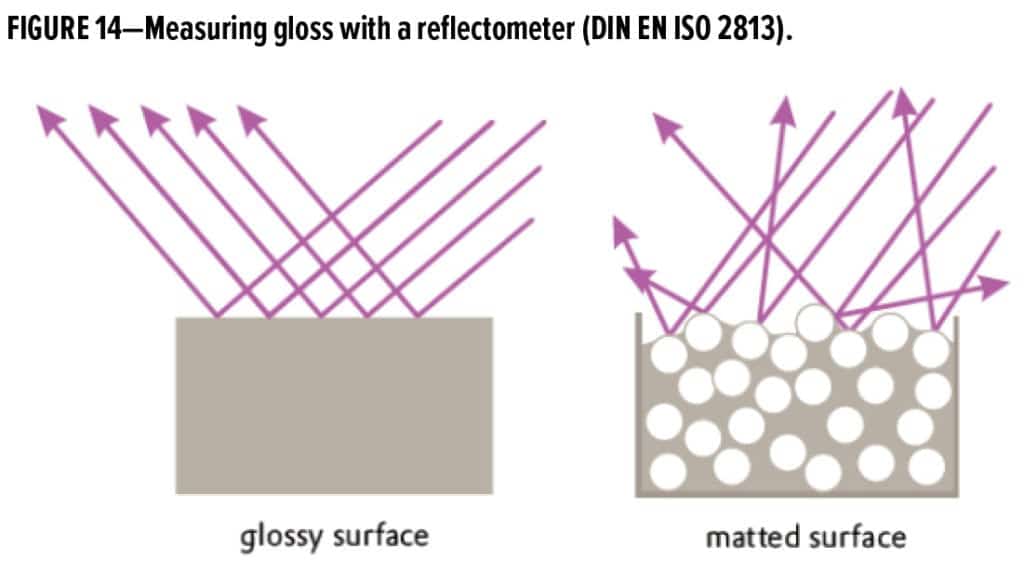
There is no common or globally accepted definition of the term “matte.” It is always measured based on a comparative measurement of the gloss against a standard.2-5 For coating surfaces, the term “gloss” means almost complete reflection in the sense that the surface reflects and scatters incident light in a wide-angle cone. The greater the cone angle, the less gloss is generally observed (Figure 14).6
Lin and Biesiada demonstrated that matting is a function of both the silica particle size and degree of coating shrinkage during drying (either through solvent evaporation, chemical reaction, or coalescence).7 Larger particles are more efficient at reducing gloss as a function of silica dosage, but the larger particles can lead to a rough surface and increased dirt pickup over time. Most silica grades used for matting coatings are produced via wet and gas phase processes and are classically larger than the grades used for rheology. Some of these grades can influence thickening to a lesser or greater extent. Surface treatment, either with wax or reactive oligomers, can help reduce viscosity build-up, prevent hard settling, and improve transparency and stability. Recent technology developments have produced silicas with improved haptic effects like soft-feel.
Recent Developments in Silica Technology
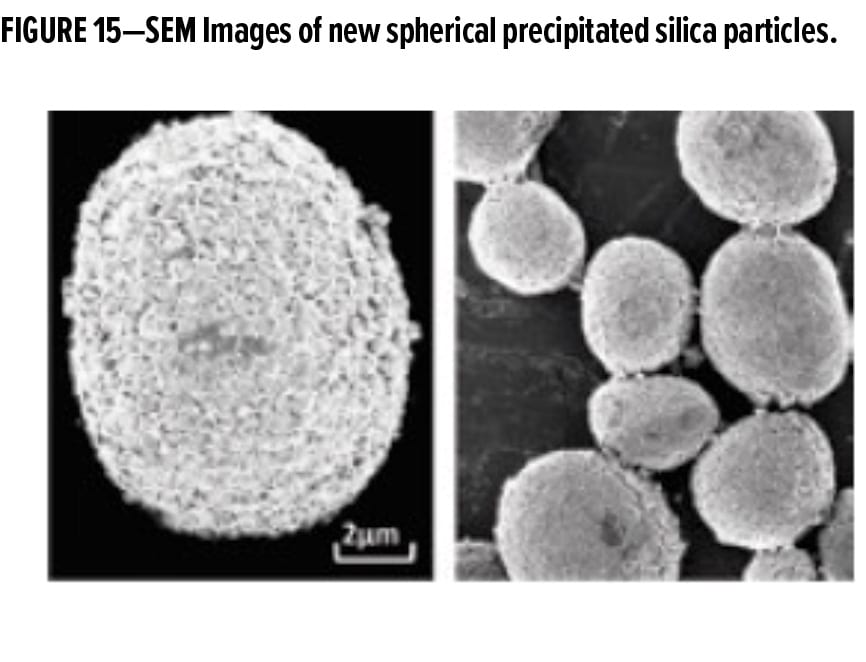
While considered a mature technology, the use of silica in coatings continues to benefit through innovation. Romer described a new process for producing precipitated silica that allows greater control of particle morphology during the precipitation process to produce highly spherical particles with narrow-sized distributions (Figure 15).8 The spherical shape imparts high apparent hardness to improve scrub, abrasion, and burnish resistance of the formulated coating, with low binder demand and minimal impact on coating rheology. These spherical silica particles also can provide matting properties, depending on particle size, and they have excellent transparency for use in deep colors and clear coats.
Both hydrophilic (200 m2/g) and some hydrophobically treated grades (e.g., DDS, HDS, HMDS, 130–300 m2/g) of fumed silica can be used in water-based coatings when it is possible to adequately disperse the powder into water dispersible resins and solutions. However, the low viscosity and high dielectric constant makes water a poor grinding medium for fumed silica, and it is difficult to achieve the degree of de-aggregation and dispersion of particles needed to achieve optimum benefits in water-based coatings. Incorporation of hydrophobically treated grades can also be difficult due to the poor wetting properties of water-based formulations, especially when resin solids drop below 35% non-volatile content. Additives, such as acetylenic diols, can help to improve the wetting and dispersion of fumed silica into water, but care must be taken to ensure that the additives do not disrupt the silica network formation, reducing rheology control.
When adding the silica directly into water, or when using a shear-sensitive, film-forming resin, it is recommended to use a pre-dispersed form of fumed silica to overcome these challenges. A new aqueous dispersion of a functionalized fumed silica has been developed using a new production process and carefully selected additives. The silica is already dispersed, so it can be easily stirred into water-based formulations without requiring high shear dispersion.
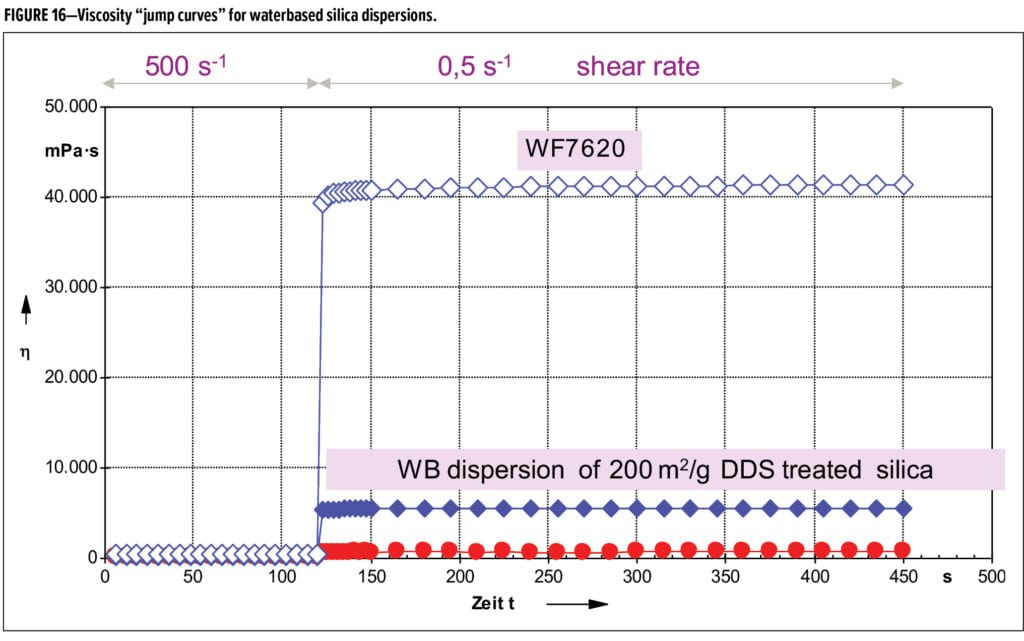
The new dispersion WF 7620, contains 20% functionalized silica with a high surface area of 300 m2/g and demonstrates outstanding rheological effectiveness in waterborne coatings, especially those applied via spray application where anti-sagging properties are critical while maintaining excellent flow and levelling properties.
This is demonstrated in the jump-curve rheology graph shown in Figure 16. The jump curve simulates a spray application where the coating is sheared at high shear (500s-1) continuously to simulate the spraying process and then the shear is suddenly reduced to 0.1s-1 simulating the coating on the substrate after application. A fast build-up of viscosity is desired to prevent sagging after application onto vertical surfaces. This enables the formulator designing perfect finishes for three-dimensional parts including general industrial coatings, transportation coatings, plastic coatings, and wood coatings. The jump curve demonstrates the new dispersion WF 7620 and gives a significant increase and improvement in rheological efficiency, compared to an existing water-based dispersion of fumed silica based on a fumed silica core of 130 m2/g. This improved performance is due to the use of a higher surface area silica, combined with a novel functionalization in-situ. The use of a higher surface area fumed silica in the dispersion also helps to improve clarity and transparency and helps to reduce haze in the final coating.
Conclusion
Modified grades of silica have been used for many years to improve a variety of performance attributes in many different coating applications. These include rheology, film formation, and mechanical properties as well as surface appearance. The morphology of the silica particles, size distribution, and surface treatment are critical to the broad range of properties that can be attained using these materials. Recent advances in the manufacturing and post-treatment processing of silica have continued to develop new grades of silica that offer new and or improved performance for the coatings industry.
References
- https://en.wikipedia.org/wiki/Silicon_dioxide.
- Ryde, J.W., Proceedings of the Royal Society (London), 131 A, 451–464 (1931).
- Brockes, A. and W. Helm, W. Farbe und Lack, 66, 53 (1960).
- Zorll, U., Farbe und Lack, 67, 426 (1961).
- Becker, Noven, H. and Rechmann, H., Farbe und Lack, 73, 625 (1967).
- H. Haussühl and H. Hamann, Farbe und Lack, 64, 642 (1958).
- Lin, B.T., and Biesiada, C., “Novel Synthetic Silica Matting Agents for Polyaspartic Coatings” Proceedings of 2016 Waterborne Symposium, 2016.
- Romer, R., “Spherical Precipitated Silica: Next Generation Particle Morphology for Performance in Coatings,” Paints and Coatings Industry, January 2017.
- “The Use of AERODISP® Fumed Silica Dispersions to
enhance Waterborne Coatings,” Evonik Technical Bulletin TI1371, September 2009.
CoatingsTech | Vol. 17, No. 6 | June 2020
