By Hong Xu, Joe Mauck, Fernanda Tavares, Anbu Natesh, and Jing Li,
Cardolite Corporation, USA
A series of zero-volatile organic compound (VOC) waterborne epoxy curing agents based on cardanol, a non-food chain and renewable biomaterial, has been developed to meet stricter regulation, as well as high-performance requirements. This article presents the latest performance studies of applying those new Cashew Nutshell Liquid-based waterborne curing agents in typical formulations for heavy duty, industrial, and transportation coatings applications. Test results revealed that the novel waterborne curing agents enable the formulations of low-VOC (< 75 g/L) direct-to-metal primer systems with excellent performance, such as balanced fast cure and long pot life, superior adhesion, and long-term corrosion protection of numerous metal substrates. Furthermore, the influence of various solid epoxy dispersions, different cure processes, and co-solvents upon film formation, adhesion, and anti-corrosion performance are reviewed, and the challenges of improving long-term corrosion protection of waterborne primer systems over galvanized steel substrates are discussed.
INTRODUCTION
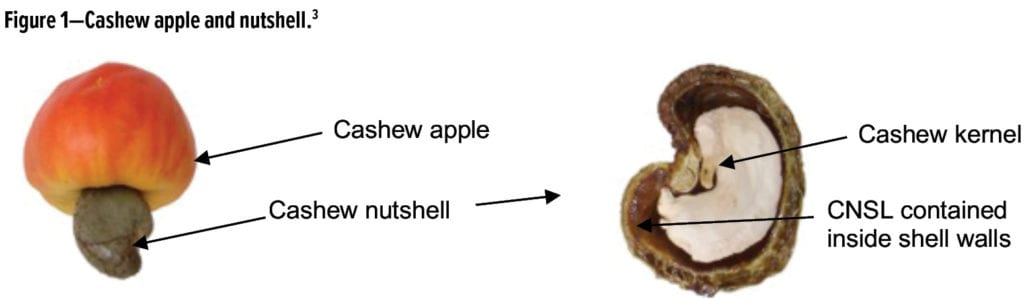
Cashew Nutshell Liquid (CNSL) is a sustainable and non-food chain biomaterial that can be obtained as a byproduct of the cashew industry.1 CNSL is contained in the honeycomb structure of the cashew nutshell (Figure 1), and it is primarily composed of 60% to 70% anacardic acid, 10% to 20% cardols, 3% to 10% cardanols, and 2% to 5% 2-methylcardols.2
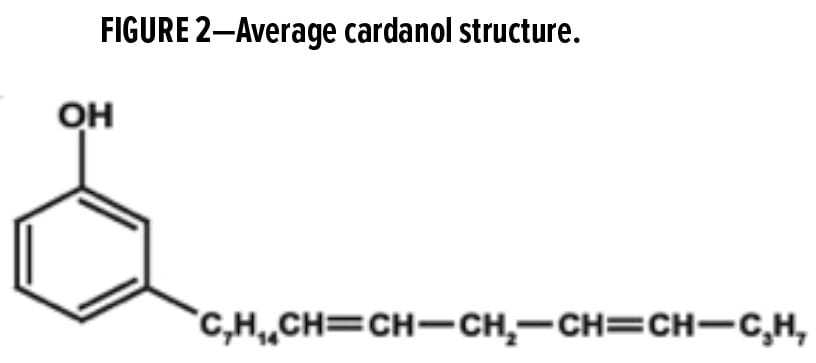
Cardanol is the main component derived from CNSL via decarboxylation and extraction.1 The chemical structure of cardanol is a pentadecadienyl phenol with a long aliphatic side chain that usually consists of a mixture of one, two, or three double bonds (Figure 2). The unique and versatile structure of cardanol enables this natural oil to become a very important chemical building block for numerous bio-based products. For example, phenalkamines and phenalkamides are derived from cardanol and as a result, those products inherit some distinctive features that benefit the final coating or adhesive systems: cardanol’s long aliphatic side chain delivers excellent water resistance (hydrophobicity), flexibility, and low viscosity; its aromatic ring provides good chemical resistance; its phenolic hydroxyl contributes to excellent adhesion to various substrates, as well as fast ambient and low-temperature cure.4
Traditionally, phenalkamines and phenalkamides are used in solventborne and high solids epoxy coating systems in marine and protective coating applications.4 To meet more stringent government regulations and the increasing requests for renewable and sustainable products, a series of zero-VOC waterborne CNSL-based curing agents was developed by stabilizing the cardanol-
based structures in water without the help of co-solvents.5 Those waterborne CNSL-based curing agents not only have high bio-content (41%~55%),6 but also retain the unique performances from solventborne phenalkamine counterparts, such as fast cure, good early water resistance, excellent adhesion to various substrates, and high mechanical strengths.5 Those CNSL-based waterborne curing agents have been successfully used in waterborne zinc-rich primer systems and mid-coat systems for the applications of container coatings and industrial coatings, as well as floor primers.5,6 To further improve the long-term (>1000 h of salt-spray exposure) corrosion protection properties over different types of metal substrates, a series of new-generation zero-VOC waterborne curing agents
has been recently developed. Some of them are still CNSL-based with high bio-content, and some of them are synthesized through non-CNSL processes to expand end-use applications.
This article presents the performance evaluation of two new waterborne curing agents, one CNSL-based and one non-CNSL type, used in several low-VOC waterborne primer systems. The advantages of these two new waterborne curing agents, such as their ease of application, good anti-corrosion performance, adhesion to various substrates, and quick wet-on-wet recoat with polyurethane (PU) are reported. Furthermore, the influences of varying solid epoxy dispersions, stoichiometric ratios, and co-solvents on the final performance of waterborne coating systems are discussed.
MATERIALS AND EXPERIMENTAL
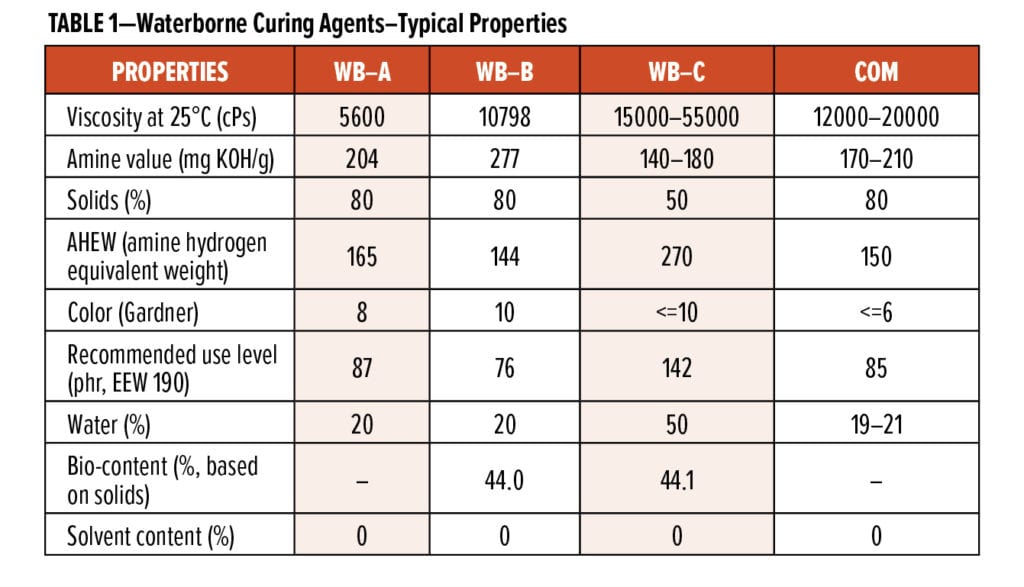
The typical properties of two new waterborne curing agents, referred to as WB-A (a modified polyamine-type waterborne curing agent) and WB-B (a CNSL-modified polyamine-type waterborne curing agent), of the first-generation CNSL-based waterborne curing agent, referred to as WB-C, and of a commercial waterborne curing agent, referred to as COM (a formulated polyamine adduct-type waterborne curing agent) are listed in Table 1 for comparison purposes.
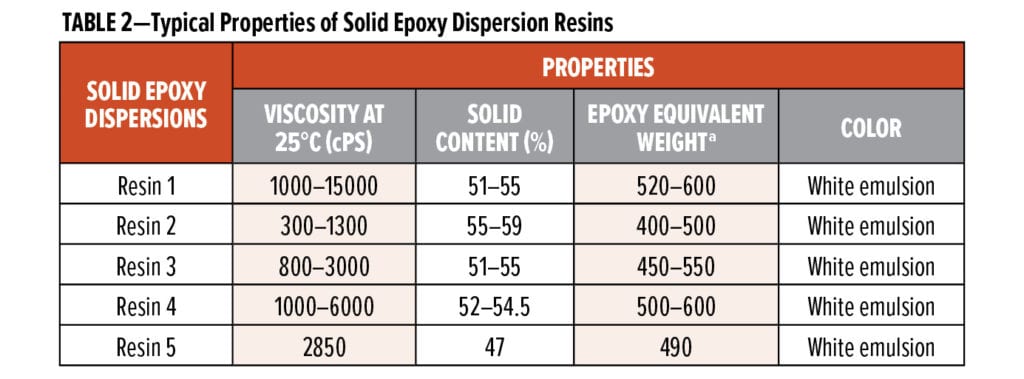
In this study, five types of solid epoxy dispersion resins, referred to as Resin 1, Resin 2, Resin 3, Resin 4, and Resin 5 were used. Their typical properties are listed in Table 2.
Linear dry time tests were carried out according to ASTM D5895-03. Clear (nonpigmented) coating systems were applied on 12 x 1 x 0.125 in. glass strips via an 8-mil (200 μm) drawdown bar. The glass strips were immediately placed on drying recorders that had been stored in a 25°C incubator. The styluses were lowered onto the wet coating to start the linear dry time tests.
The waterborne primer systems were applied over different types of substrates via air spray. After a seven-day room temperature (RT) cure or bake in a 60°C oven for one to two hours, the panels were evaluated for adhesion tape tests (ASTM D3359) and salt-spray exposure (ASTM B117).
RESULTS AND DISCUSSION
Part I: Improved Application Properties From New Waterborne Curing Agents
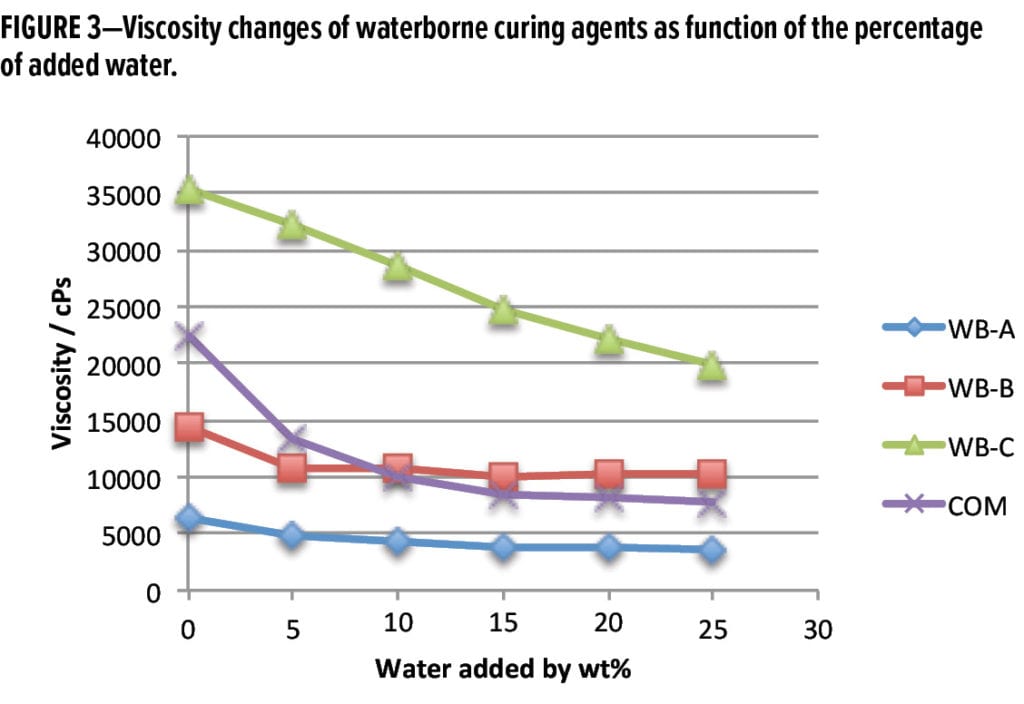
WB-C is part of the first generation of CNSL-based waterborne curing agents, and it has 44.1% calculated bio-content. In previous studies,5,6 WB-C systems exhibited very interesting properties, such as fast cure, excellent early water resistance, and excellent compatibility with various epoxy resins. However, WB-C systems needed further improvements on some application properties, such as an ability to be easily diluted by water and a more balanced cure speed and pot life. WB-A and WB-B are the latest high-performance waterborne curing agents to be developed. WB-A is not based on CNSL, while WB-B is CNSL-based with 44.0% calculated bio-content. In Table 1, it can be seen that, even though WB-A (5600 cPs) and WB-B (10798 cPs) curing agents are supplied at 80% solids, they still have much lower viscosities than 50% solids WB-C (15000–55000 cPs). The viscosities of WB-A and WB-B are also lower than that of a commercial product, COM.
If the initial solids differences of the four waterborne curing agents are ignored and the focus is on the viscosity changes of each waterborne curing agent after adding a certain percentage of water, it can be seen in Figure 3 that WB-A and WB-B exhibit improved dilution properties: only 10% water could lower the viscosities of WB-A and WB-B from 6300 cPs and 14700 cPs to 4200 cPs and 10000 cPs, respectively; while the viscosity of WB-C system was kept around 20000 cPs after adding 25% water. The results suggest that the two new waterborne curing agents are more application-friendly.
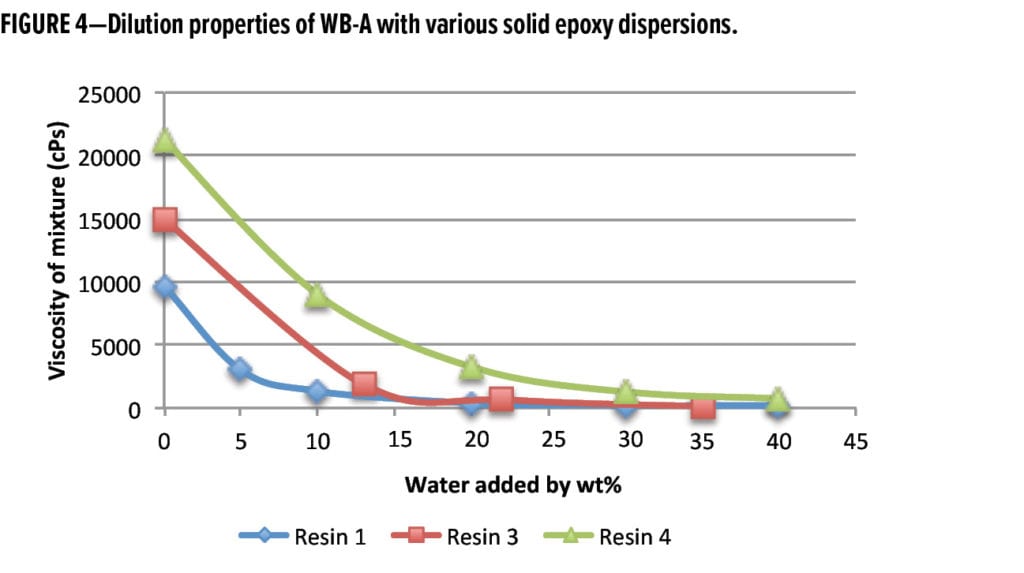
It is common for a waterborne curing agent to have different initial viscosities when mixed with various solid epoxy dispersion resins. For example, the initial viscosities of WB-A mixed with Resin 1, Resin 3, and Resin 4 (at stoichiometric ratio) were 9650 cPs, 14920 cPs, and 21250 cPs, respectively, as shown in Figure 4. Regardless of the resin selected, the dilution results indicate WB-A-based systems can be easily diluted to low viscosities with small amounts of water. Less than 15% extra water was enough to bring admixture viscosities of WB-A with Resin 1 and 3 to less than 2,000 cPs. Even in the case of the high admixture viscosity of WB-A with Resin 4, 20% extra water could effectively reduce viscosity to around 3000 cPs. This good dilution property of WB-A suggests that WB-A is suitable for high solids (>60% solids) waterborne formulations.
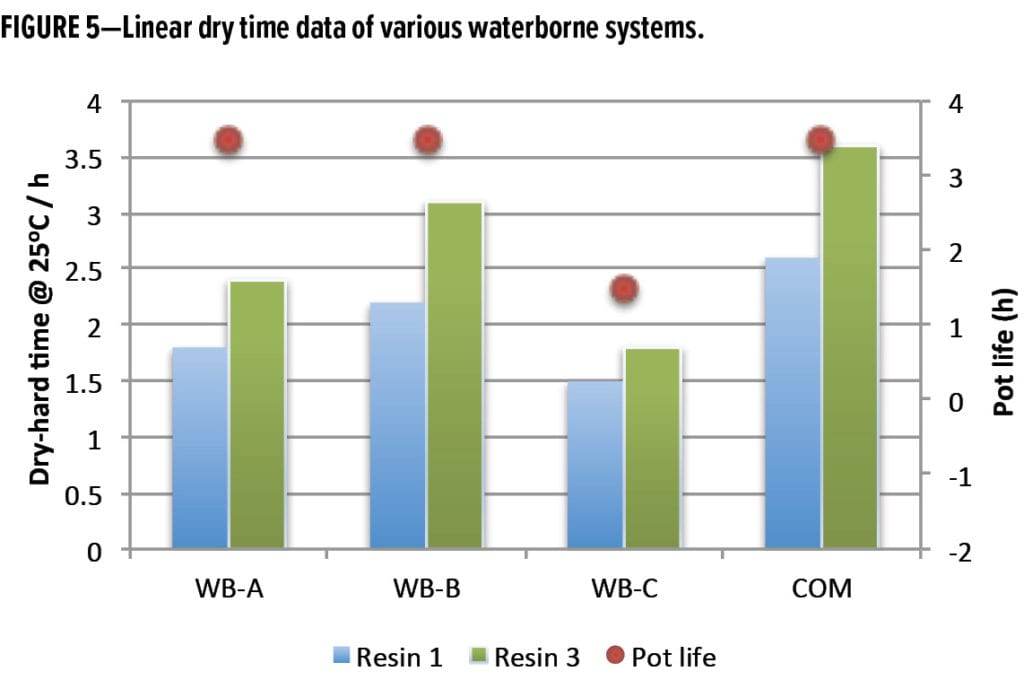
It is always desirable that a paint system exhibits fast-cure performance while having a long work window (pot life) for easier application, though this is usually a difficult task to accomplish. For example, CNSL-based WB-C curing agent has excellent fast-cure properties (less than 2 h of dry-hard time at 25°C) with various solid epoxy dispersion resins. However, as shown in Figure 5, its average pot life is around 1.5 h (displayed as red dot), which can be considered short for some applications. Through better design of the polymers’ structures, new waterborne curing agents WB-A and WB-B exhibit the improved properties with fast cure and longer pot life. As indicated in Figure 5, WB-A and WB-B could achieve dry-hard times of 3 h or less with both Resin 1 and Resin 3 while providing extended pot lives of 3.5 h. The competitive-based system (COM) also shows long pot life but was slower in dry-hard times.
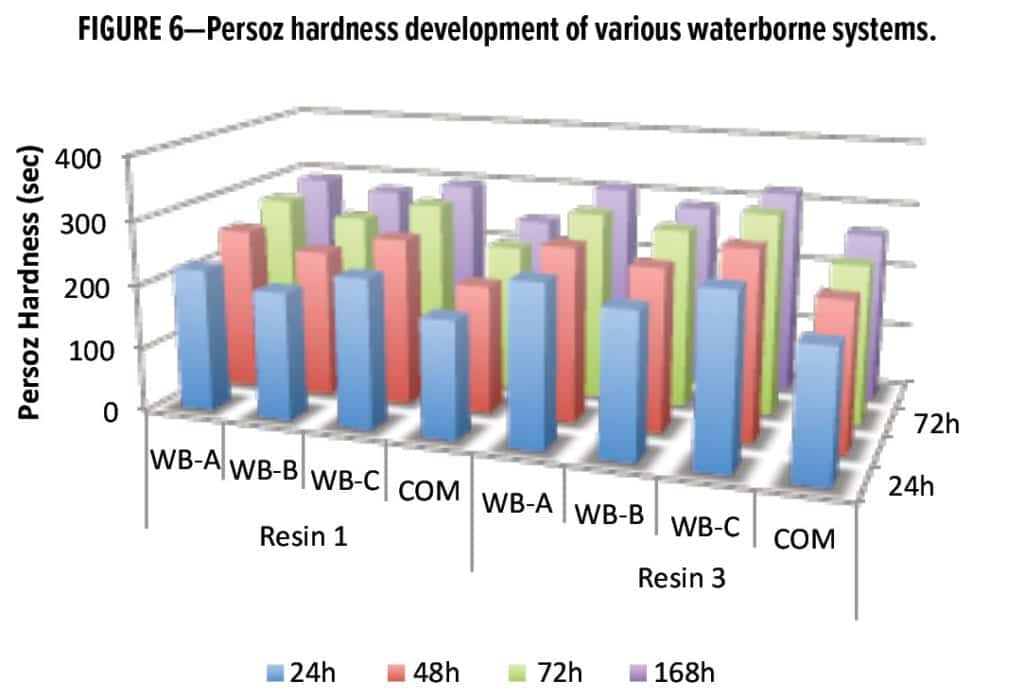
The Persoz hardness data from Figure 6 confirm that WB-A and WB-B systems deliver faster hardness development than COM-based systems. Moreover, the fast-cure properties of WB-A and WB-B could benefit wet-on-wet topcoat applications, as presented in detail later in this article.
Part II: Waterborne Primer/Mid-coat Systems Based on New WB-A and WB-B Curing Agents
Excellent long-term corrosion protection is a key property required in waterborne epoxy primer systems and mid-coat systems; however, it is also the most challenging to achieve. In this study, newly developed WB-A and WB-B curing agents were evaluated to assess their anti-corrosion performance after extended salt-spray exposure. Different performance aspects, such as rust or blisters on field, and creep along the scribe line, were checked after certain exposure intervals, and wet adhesion was also conducted on the test panels after 800 h of salt-spray exposure.
In Table 3, the formulations of three low-VOC waterborne primer systems, MC #1, MC #2, and MC #3, were listed based on WB-A, WB-B, and WB-C curing agents, respectively. For comparison purposes, all three primer systems were formulated with the same solid epoxy dispersion (Resin 3), at similar solids (around 57%), with comparable pigment volume concentration (PVC from 27% to 30%), and using the same 1.25 stoichiometric ratio. It can be seen that VOC values for those waterborne primer systems are less than 75 g/L.
The anti-corrosion performances of MC #1, MC #2, and MC #3 formulations were assessed in direct-to-metal (DTM) primers. Those primer systems were directly air sprayed to various nonpretreated metal substrates, such as SA 2.5 blasted steel panels, cold rolled steel (CRS) panels, galvanized steel panels, aluminum alloy AA 2024 T3 panels, and stainless steel panels. Two cure conditions were used for those waterborne primer coated panels: seven-day RT cure or one- to two-hour bake in a 60°C oven. The final dry film thickness (DFT) of the waterborne primer films after cure was around 55 to 80 μm. Test panels were taped or coated on the back and edges before being placed into the Q-Lab Q-FOG chamber for ASTM B-117 test.
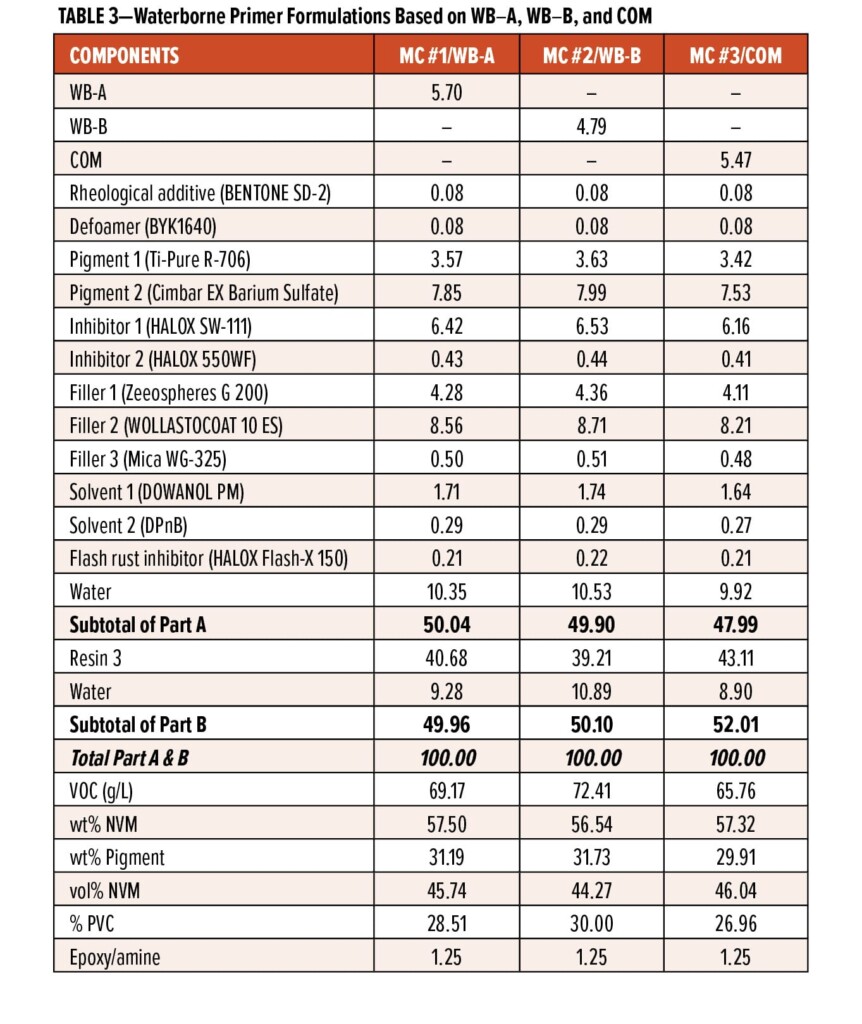
Figure 7 shows the panel images of MC #1, MC #2, and MC #3 systems after about 1000 h of salt-spray exposure and after an 800-grit sandpaper was used to remove surface rust stains. Those films were applied over SA 2.5 sand-blasted steel substrates with about 60 to 75 μm DFT. It can be seen that only some small blisters formed near the scribe lines of the WB-A system, and the coating still has very good adhesion to the steel substrate along the X-shaped scribe lines. The WB-B system has smaller blisters in comparison to the WB-A system, but that might be due to 200 h less salt-spray exposure time. The COM system also shows good anti-corrosion performance, with very few blisters formed on the film surface, but some creeps observed along the scribe lines.
As part of this study, one can also notice the significant impact of film thickness on long-term anti-corrosion performance, especially over sand-blasted panels. For example, MC #1 formulation was applied to panels at various DFT of 37 μm, 50 μm, 65 μm, and 100 μm, and then exposed in the salt-spray chamber. After 500 h of salt-spray exposure, the system with 37 μm DFT already showed severe rust and blisters, while the other three systems still had intact films; up to 1100 h, the system with 50 μm DFT exhibited more dense blisters along the scribe line than the system with 65 μm DFT, but the system with 100 μm DFT showed no blisters. As expected, higher film thickness provides better and longer anti-corrosion protection to metal substrates. In addition, the test results in this study suggest that the impact of film thickness of waterborne primer systems on anti-corrosion performance could become more significant over sand-blasted steel panels. That is probably because waterborne primer systems tend to penetrate and settle in the bottom crevices of the rough surface of sand-blasted steel panels, which results in some weak areas with much lower film thickness where corrosion can start.
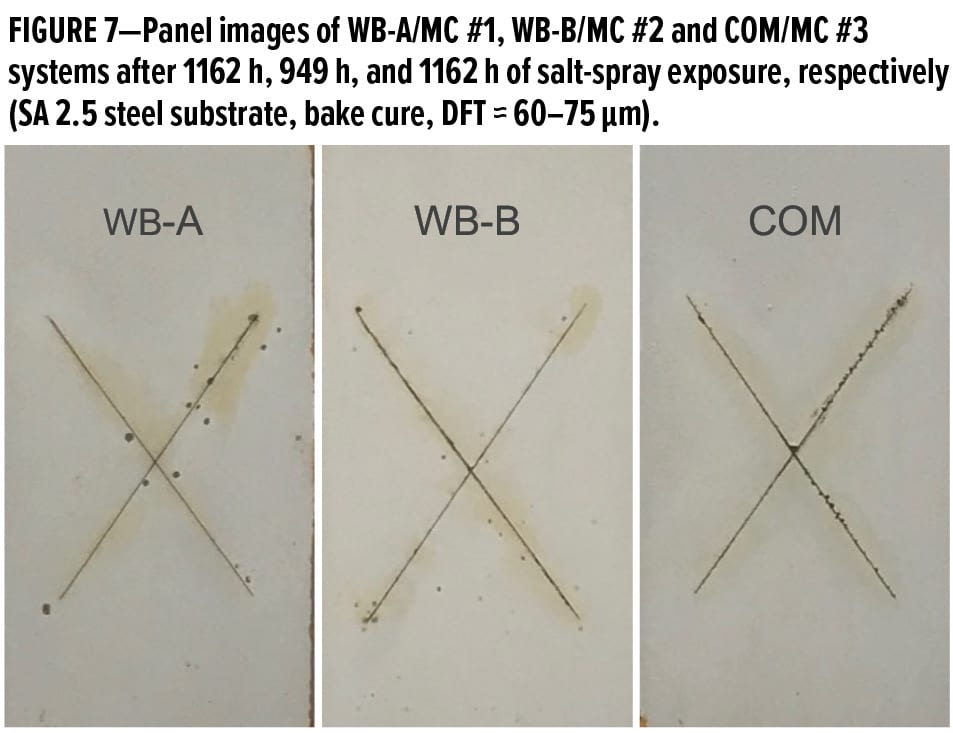
Figure 8 exhibits the panel images of MC #1, MC #2, and MC #3 systems after about 1000 h of salt-spray exposure and after removal of surface rust stains with an 800-grit sandpaper. Those waterborne primer systems were applied on CRS panels with DFT of about 75 μm. Blisters were only observed along the scribe lines for all three systems, but the adhesion of coating films to CRS substrates along the scribe lines was not excellent; some underneath creeps developed with the widths of 3 mm, 1.5 mm, and 3.5 mm for MC #1, MC #2, and MC #3 systems, respectively. These test results indicate the new waterborne primer systems could provide very good corrosion protection on CRS, but it is still a major challenge to achieve superior adhesion to steel substrates with a smooth surface profile after long-term salt-spray exposure.
Next, this study evaluated the anti-corrosion and adhesion properties of the new waterborne curing agents on various metal substrates commonly used in some industrial coatings applications that include aluminum alloys, stainless steel, and galvanized steel. In general, good adhesion, and therefore long-term corrosion protection to these substrates, could be difficult to achieve, especially with low-VOC formulations.
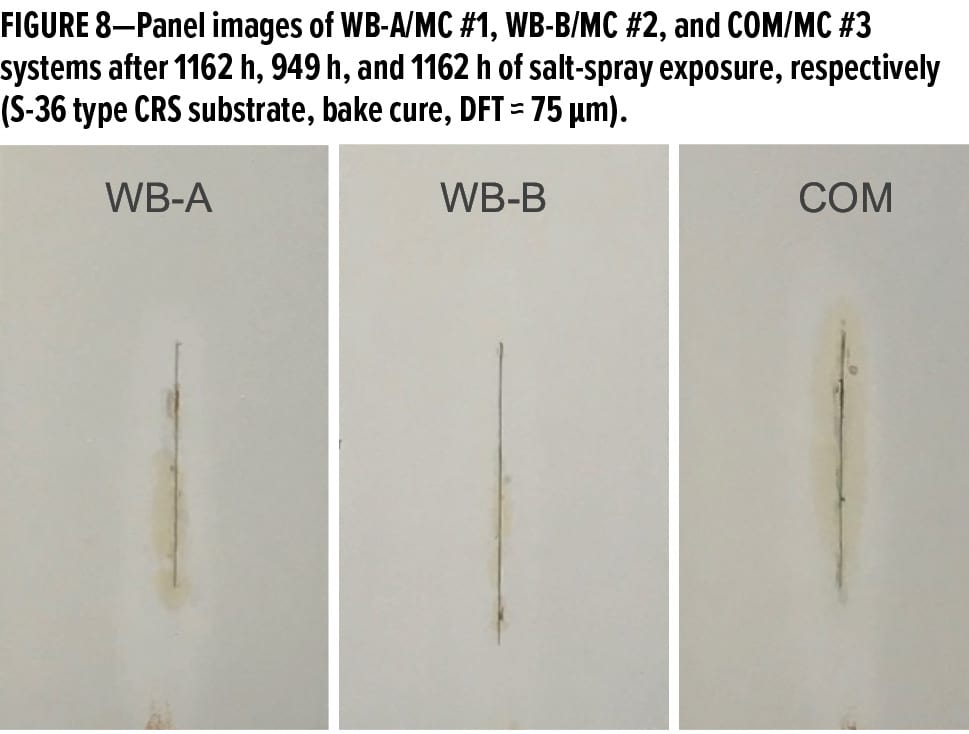
Figure 9 displays the panel images of MC #1, MC #2, and MC #3 systems over aluminum alloy AA 2024 T3 substrates after 2018 h, 1852 h, and 2018 h of salt-spray exposure, respectively. (The panel surfaces were sanded via 220-grit sandpaper followed by an acetone rinse and paper towel cleaning.) It can be seen that MC #1 and MC #3 systems exhibited excellent protection properties with only a few very tiny blisters formed along the scribe line after 2018 h of salt-spray exposure; the MC #2 system also presented good anti-corrosion properties with no blisters and delamination after 1800 h of salt-spray exposure on the panel field, though some small blisters formed along the scribe line. Wet adhesion was measured on the test panels (top right side of each panel) that had been exposed in the salt spray chamber to endure the continuous attacks of ions and water for more than 800 h; therefore, the excellent wet cross-hatch adhesion observed from three primer systems over AA 2024 T3 indicates excellent long-term corrosion protection.
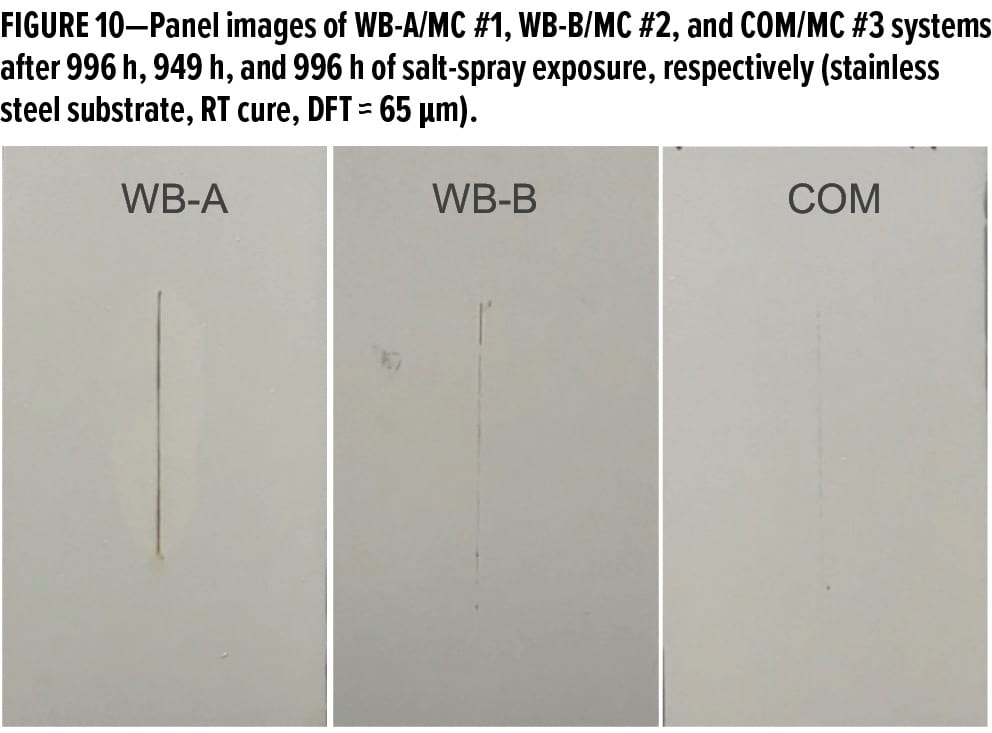
The panel images of MC #1, MC #2, and MC #3 systems applied over stainless steel substrates after about 1000 h of salt-spray exposure are shown in Figure 10. There were no blisters or delamination observed for the three systems.
Furthermore, MC #1, MC #2, and MC #3 systems were also applied over galvanized steel substrates that were simply wiped with acetone. Figure 11 displays the panel images of those systems after about 1000 h of salt-spray exposure: MC#1 system had two to three large blisters and medium dense blisters with size of 6 to 8 along the scribe line; MC #2 system showed two large blisters and some blisters with size of 8 along the scribe line; and MC #3 system formed much larger and denser blisters from the center scribe line to both sides.
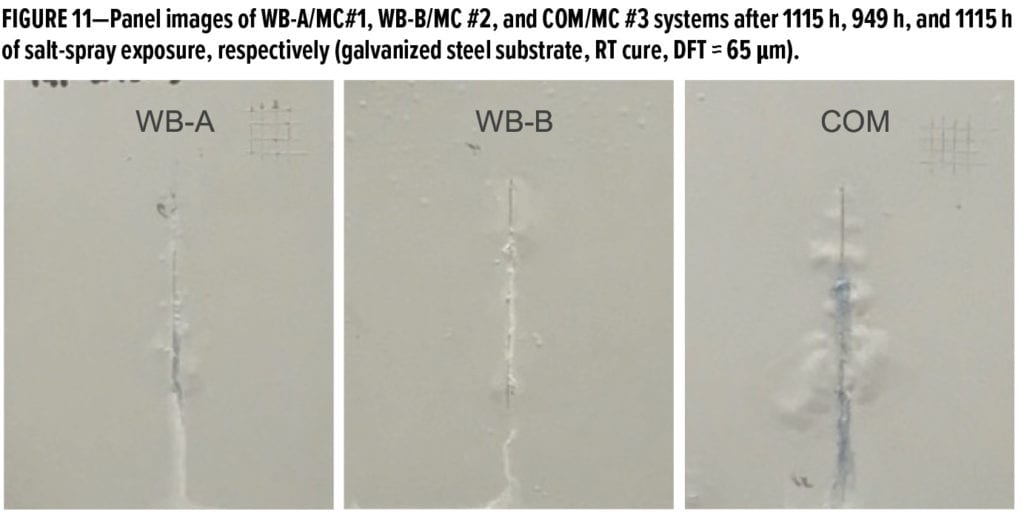
Results demonstrate that in comparison to other substrates evaluated in this study, the blisters formed on galvanized steel panels were much more severe for similar duration of salt-spray exposure. Even though galvanized steel was the most challenging substrate, MC #1 and MC #3 systems still exhibited fairly good wet adhesion performance and could be good options for formulators. Some formulation study to improve the corrosion protection on galvanized steel substrate is reported in Part III.
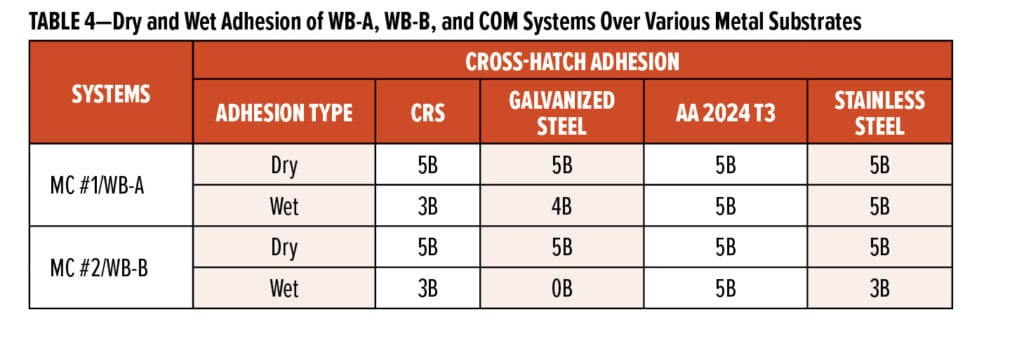
Table 4 summarizes the dry and wet cross-hatch adhesion of MC #1 and MC #2 primer systems over four types of metal substrates: nonpretreated bare CRS with a smooth mill finish, galvanized steel wiped with acetone, AA 2024 T3 panels sanded via 220-grit sandpaper followed by acetone rinse and wiped, and stainless steel with no surface preparation. The dry adhesion values were obtained on the cured panels that were not exposed to salt spray, while the wet adhesion values were measured on panels after exposure in the salt spray chamber for more than 800 h. It can be seen that MC #1 and MC #2 primer systems had very good dry adhesion regardless of the type of metal substrate used; after being exposed to salt spray for more than 800 h, the wet adhesion of MC #1 and MC #2 systems over aluminum alloy substrate was still excellent. Over stainless steel substrate, MC #1 system still maintained excellent wet adhesion while MC #2 system showed a little drop to 3B. Both MC #1 and MC #2 systems still achieved 3B wet adhesion over bare CRS. The major difference in performance between MC #1 and MC #2 systems was observed when applying over galvanized steel substrates: MC #1 system still provided good wet adhesion of 4B, but MC #2 system lost adhesion after long salt-spray exposure. The test results in Table 4 demonstrate that the two new waterborne samples could provide excellent dry and wet adhesion over various metal substrates, except MC #2 system showed poor wet adhesion over galvanized steel substrate. The excellent adhesion properties of new waterborne samples could further benefit the long-term corrosion protection of different metal substrates.
Part III: Wet-on-Wet Properties
Quick self-recoat or recoatability with PU coatings is a highly desirable property in industrial applications, such as transportation coatings, agricultural, construction and earth-moving equipment coatings, and rail car coatings. Very short recoat intervals between the application of primer and the next coating layer are required, such as 30 min or less at RT or elevated cure conditions. Since the primer may not be fully dried when the next coat is applied, this cure process is usually called wet-on-wet application. If a primer system is slow in cure or has poor compatibility with PU topcoat, it will most probably result in a dieback issue, which means that the cured PU topcoat no longer has its original high gloss and may show poor adhesion to the primer system.
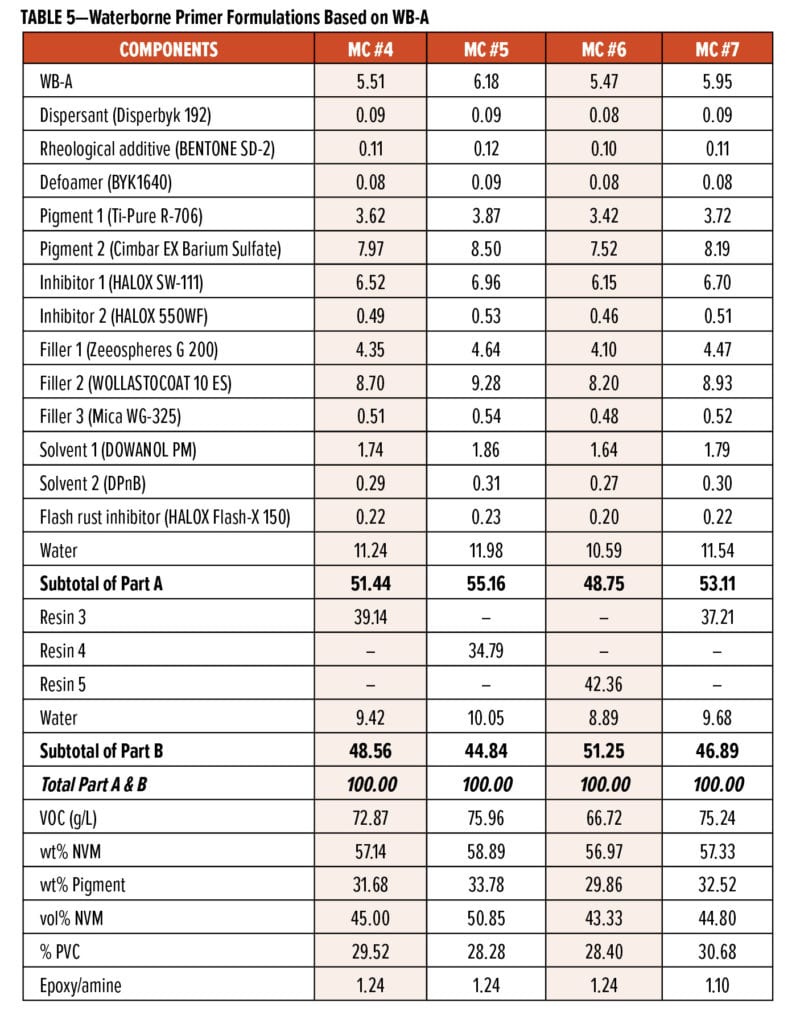
The wet-on-wet topcoat performances were evaluated on the MC #4 system based on WB-A curing agent and Resin 3; the MC #4 primer system had VOC of less than 75 g/L. The primer system was applied via air spray over CRS substrate at a wet film thickness of 50 to 65 mm. The test panels were set up in six groups: the panels in Groups #1 to #3 were cured at RT for 15, 30, and 45 min, respectively. Meanwhile, the panels in Groups #4 to #6 were baked in a 60°C oven for 15, 30, and 45 min, respectively. Subsequently, a commercial 2K solventborne PU system was applied over all six groups of panels via air spray. After a 24-h RT cure, the glosses of the PU top-coated panels, as well as their adhesions between the primer and PU topcoat, were measured and are listed in Table 6.
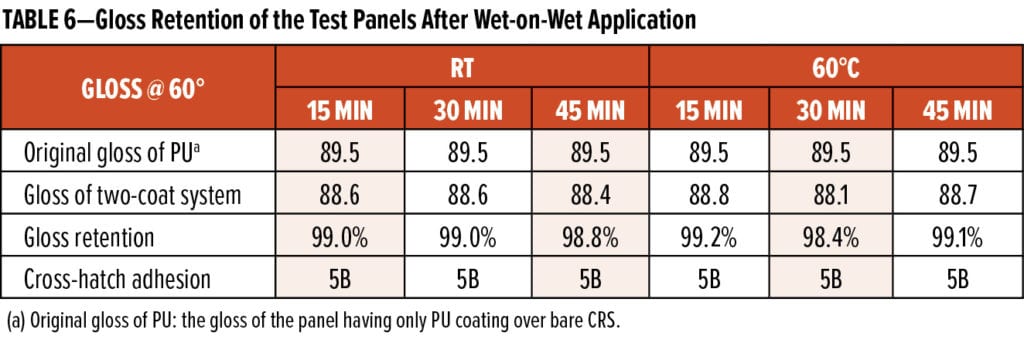 Photo images of those panels after cross-hatch adhesion tests are shown in Figure 12. Those test results reveal that MC #4 system could maintain high gloss retention (>98%) and excellent adhesion (5B) to the commercial PU topcoat regardless of the cure conditions and recoat intervals. Furthermore, panels from Group #1 to #3 were tested in salt spray for 881 h, and they showed no blisters on field and had creeps along scribe lines of less than 2 mm (shown in Figure 13). The wet-on-wet applied panels confirmed that the adhesion between MC #4 and PU topcoat was excellent even after a long time of salt spray testing. These combined test results suggest that WB-A systems are suitable for industrial coating applications due to their quick wet-on-wet properties and excellent long-term corrosion protection performance.
Photo images of those panels after cross-hatch adhesion tests are shown in Figure 12. Those test results reveal that MC #4 system could maintain high gloss retention (>98%) and excellent adhesion (5B) to the commercial PU topcoat regardless of the cure conditions and recoat intervals. Furthermore, panels from Group #1 to #3 were tested in salt spray for 881 h, and they showed no blisters on field and had creeps along scribe lines of less than 2 mm (shown in Figure 13). The wet-on-wet applied panels confirmed that the adhesion between MC #4 and PU topcoat was excellent even after a long time of salt spray testing. These combined test results suggest that WB-A systems are suitable for industrial coating applications due to their quick wet-on-wet properties and excellent long-term corrosion protection performance.

Part IV: Formulation Study
In this study, the influences upon anti-corrosion performance of changing some factors in formulations, such as varying solid epoxy dispersions, using different stoichiometric ratios, or adding different co-solvents, were evaluated.
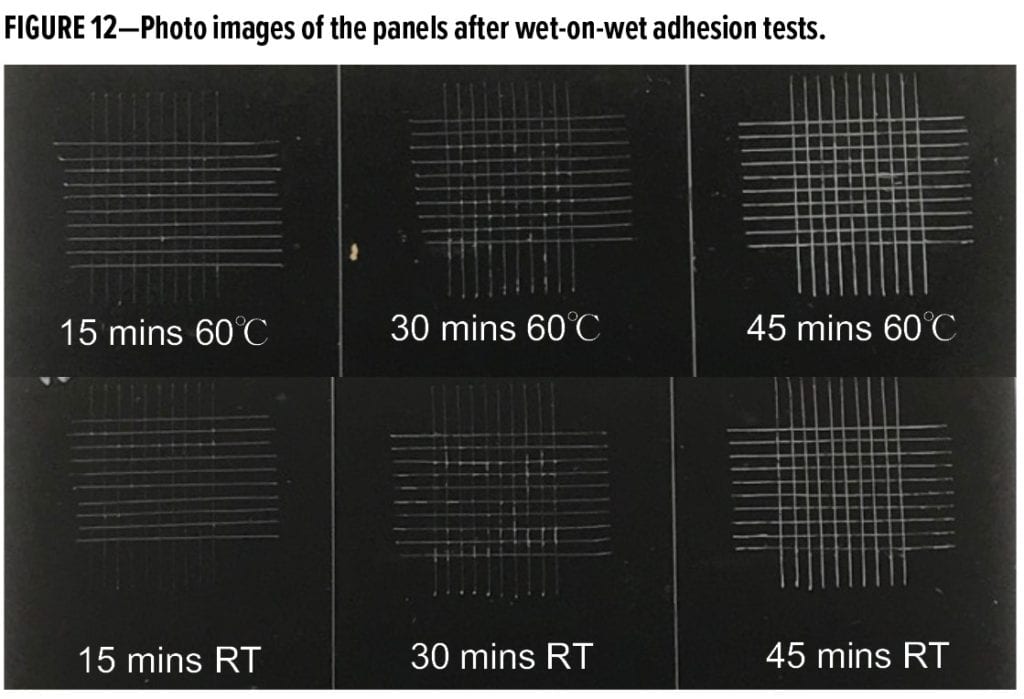
In waterborne coating formulations, good compatibility between solid epoxy dispersion and waterborne curing agent is critical for good overall performance. In this study, three primer systems, MC #4, MC #5, and MC #6, were formulated at very comparable pigment composition and PVC, at similar solids % and co-solvent content, and by using the same waterborne curing agent WB-A at 1.24 stoichiometric ratio. Only the solid epoxy dispersions were changed with Resin 3, Resin 2, and Resin 5 being used for MC #4, MC #5, and MC #6 systems, respectively. The test results revealed that the primer systems of MC #4 (based on Resin 3) and MC #5 (based on Resin 2) exhibited much better corrosion protections than the MC #6 system (based on Resin 5), regardless of the types of metal substrate. For example, the three panels shown in Figure 14, representing MC #4, MC #5, and MC #6 systems from left to right, were applied over galvanized steel substrate. After 750 h of salt-spray exposure, MC #4 and MC #5 systems had some blisters along the scribe lines, but no cracks or blisters on the fields. However, the coating film of MC #6 system became brittle, and some areas started to show delamination from the substrate. Those test results confirm that choosing the proper solid epoxy dispersion has significant effects on the final performance of waterborne primer systems.
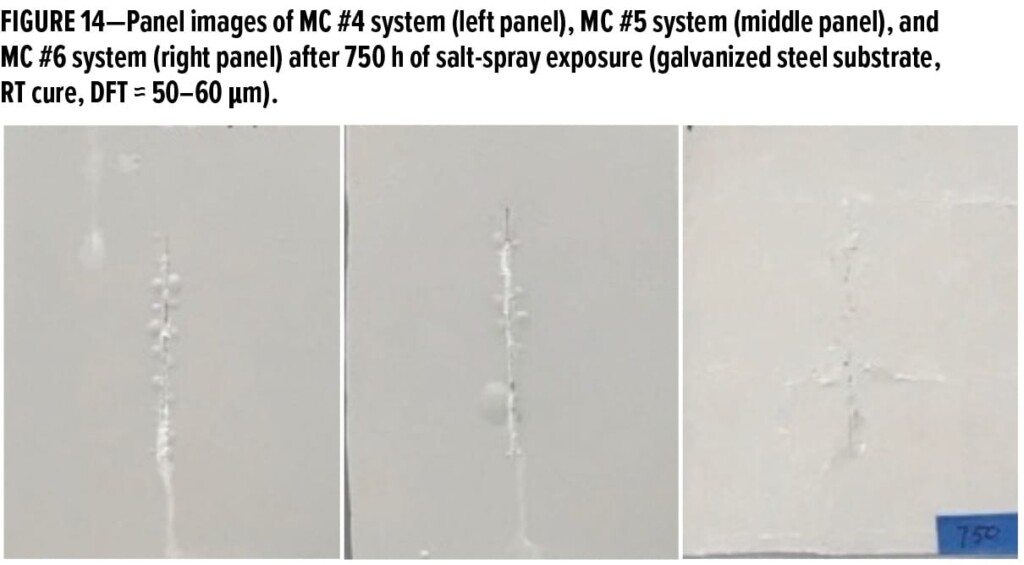
Besides the epoxy resin selected, the stoichiometric ratio between epoxy and amine has a noticeable influence on the overall properties of waterborne primer systems. In our previous studies,5,6 it was found that the stoichiometric ratio of 1.25 gave the best performance when waterborne primers were applied over blasted or CRS substrates. Test results in Part II confirmed that excellent long-term corrosion protection could be obtained for steel substrates with 1.25 stoichiometric ratio but was somehow worse over galvanized steel substrate. Therefore, two primer systems, MC #4 and MC #7, with different “epoxy to amine” stoichiometric ratios of 1.25 and 1.10, respectively, were tested over galvanized steel substrates. After 750 h of exposure in salt spray chamber, panels shown in Figure 15 suggested that MC #7 system with stoichiometric ratio of 1.10 exhibited better anti-corrosion performance than MC #4 due to much fewer blisters formed along the scribe line. Results indicate that the increase of WB-A use level in formulations could effectively help to eliminate the blisters over galvanized steel substrate.
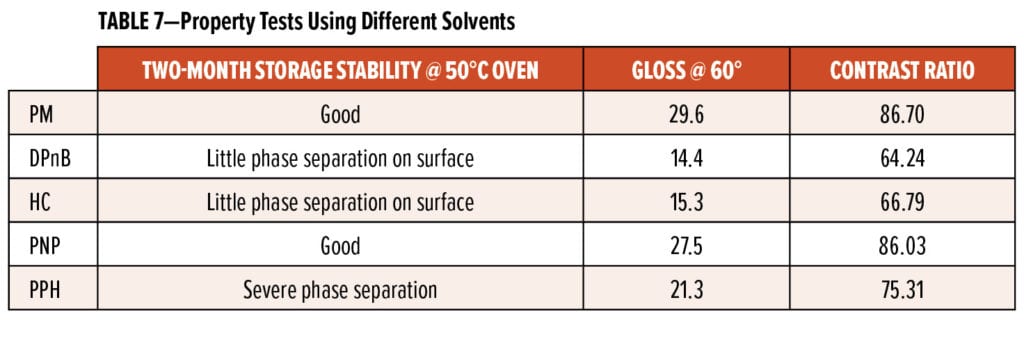
To achieve the low-VOC target of less than 75 g/L for waterborne primer systems, the amount of co-solvent used in the formulation was significantly reduced; subsequently, the answer to “which types of co-solvents could efficiently help with proper film formation even at small use level?” became important. In this study, five types of glycol ether solvents were assessed through storage stability, film appearance, and hiding power. Those solvents are propylene glycol monomethyl ether (PM), dipropylene glycol n-butyl ether (DPnB), hexyl carbitol (HC), propylene glycol propyl ether (PNP), and propylene glycol phenyl ether (PPH). The samples for storage stability tests were prepared by mixing WB-A curing agent, co-solvent, and DI water at 3:1:1 ratio, then the samples were placed into a 50°C oven for two months before any phase separation was checked. For gloss measurement, the panels were prepared by applying white waterborne primers containing various co-solvents over CRS panels. For the contrast ratio measurement, those waterborne primers were applied over Leneta sealed black paper charts via drawdown after aging for 20 h at RT. After cure, contrast ratio and gloss were measured using the Datacolor Check II Plus and BYK Gardner micro-TRI-gloss meter.
Table 7 shares storage stability, gloss, and contrast ratio results. One can see that PM and PNP systems had no phase separation at 50°C after two months, which suggests that PM and PNP have better compatibility with WB-A than the other three solvent systems. Gloss and contrast ratio data revealed similar trends: the PM and PNP systems gave higher glosses than the other three solvent systems due to better film formation. PM and PNP systems gave higher contrast ratios, which indicated those two systems had better hiding power, or ability to cover black background, as observed in Figure 16. Since those five primer systems were applied over Leneta sealed black paper charts after 20-h aging of waterborne paints at RT, the better hiding power suggests that PM and PNP could possibly extend the pot life of waterborne primer systems based on WB-A and Resin 3.
CONCLUSIONS
In this article, a series of low-VOC (<75 g/L), high-performance waterborne primer formulations based on two newly developed solvent-free waterborne curing agents were evaluated in direct-to-metal application. The results confirmed that both CNSL-based and non-CNSL waterborne curing agents could provide balanced cure properties with fast dry times and long pot life, as well as good dilution properties. Additionally, the new waterborne primer systems exhibited excellent dry and wet adhesion over various metal substrates after 800 h of salt-spray exposure, which resulted in excellent corrosion protection. Moreover, test results from the wet-on-wet PU topcoat on epoxy primer study demonstrated that the new waterborne curing agents enable short recoat intervals of 15 to 30 min for multi-layer coating systems to achieve excellent adhesion and anti-corrosion properties, as well as to maintain aesthetic appearance.
Furthermore, formulation studies that varied solid epoxy dispersions, stoichiometric ratios, and co-solvents suggested that (1) good compatibility between curing agent and solid epoxy dispersion has a significant impact on the overall performance of waterborne coating systems; (2) by increasing the use level of WB-A curing agent to solid epoxy dispersion from 0.8 to 0.9, anti-corrosion properties of waterborne primer systems could be improved on substrates like galvanized steel; (3) the right co-solvents in waterborne primer systems could not only improve the storage stability but are likely to help extend pot life.
Acknowledgments
The authors would like to express their gratitude to the R&D chemists Xiaoping Ding and Chuang Zeng of Cardolite Zhuhai, China for the new product developments.
References
- Lomonaco, D., Mele, G., and Mazzetto, S.E., Cashew Nutshell Liquid (CNSL): From an Agro-industrial Waste to a Sustainable Alternative to Petrochemical Resources, Anilkumar, P. (Ed.), Springer International Publishing AG, Cashew Nut Shell Liquid, 2017.
- Gedam, P.H. and Sampathkumaran P.S., “Cashew Nut Shell Liquid: Extraction, Chemistry and Applications,” Prog. Org. Coat., 14: 115-157 (1986).
- Gaba, E., Wikimedia Commons user: Sting https://commons.
wikimedia.org/wiki/File: Cashew_Brazil_fruit_3.png; accessed: Nov. 8, 2015. - Dai, Z., Constantinescu, A., Dalal, A., and Ford, C., “Phenalkamine Multipurpose Epoxy Resin Curing Agents,” Cardolite Corporation, Newark, NJ, reprinted from EPI-ERF Conference, Sept. 1994.
- Lawson, D.C., Hu, J., and Natesh, A., “Cashew Nutshell Liquid Based Waterborne Epoxy Curing Agent for
Concrete,” The Waterborne Symposium, Feb. 2016. - Xu, H., Natesh, A., and Tavares, F., “New CNSL-based Waterborne Curing Agents for Low VOC Zn-rich Primer and Protective Epoxy Primer Systems,” European Coating Show Conference, March 18-19, 2019.
CoatingsTech | Vol. 17, No. 5 | May 2020
