By , Covestro
Background
Is circular thinking new? When and why did linear thinking become more popular? To begin, let’s look at some history in mass production and consumerism to see how the paint and coatings industry has evolved into the supply streams seen today.
In the 1920s, a term began to be discussed within the mass production automotive industry. Alfred Sloan, a GM executive, is credited with the first use of the term “dynamic obsolescence” — a term used to describe the planned obsoletion of cars.1 The intention was to entice consumers to want to purchase newer models before the older ones were beyond their useful life. New models were designed to make the previous ones less desirable—some were even designed for a limited life. The 1923 Chevrolet is often cited as the first such example.
Later the term evolved into “planned obsolescence” and was accepted as a principle of mass manufacturing. In his 1932 essay, Bernard London proposed the idea of “Ending the Depression Through Planned Obsolescence”—an approach that drew criticism. In the 1960 book The Waste Makers, Vance Packard scrutinized the concept, calling it “the systematic attempt of business to make us wasteful, debt ridden, and permanently discontented individuals.”2
As mass production, consumerism, and convenience became part of post-World War II life, another term emerged: “Throw Away Living.” First used in an article from the August 1, 1955, issue of LIFE magazine, the term was used in the author’s “critical view of over consumerism and excessive production of short-lived or disposable items over durable goods.”3
Even so, consumers had not always been part of a “Throw Away” culture. In fact, pre-World War II thinking was more focused on not being wasteful. There was value in repurposing items for new uses. A good example of this is how flour was marketed to consumers during the post-Civil War period through the 1950s. During this time, textiles were used to replace barrels in the transportation of dry goods. It was common for the consumer, who did not want to waste, to make clothing and other articles from the leftover flour and feed sacks. Manufacturers took note of the trend and began to print attractive patterns on their packaging, an example of public-desired reuse. They also printed their product branding in washable ink and gave the consumer instructions on how to remove it, illustrating manufacturer’s design for reuse. Once the clothing was no longer useful, the textile was again repurposed into quilts and rag rugs, showing an end-of-life repurpose.

Circularity in the Chemical Industry
Maria Morais, in The Future of Commerce, states that “91% of the global economy is not circular and that this represents a $4.5 trillion profit opportunity for the industry.”4 Chemical industry CEOs such as Markus Steilemann of Covestro have recognized that there is value in everything we generate. Steilemann has publicly stated many times that “We will be fully circular.” He says to achieve this goal there are four key pillars to realizing a circular economy:
- alternative raw materials
- innovative recycling
- joint solutions
- use of energy
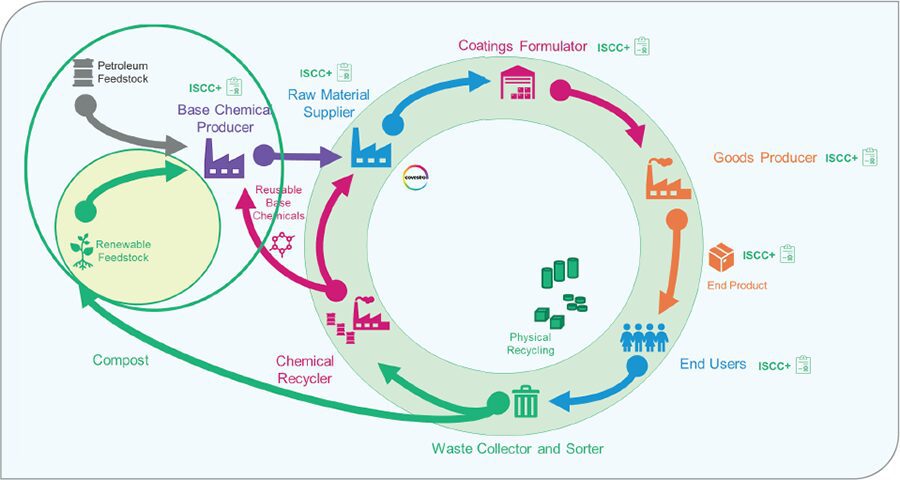
As shown in Figure 1, there are many places where circularity can be brought into the value chain. First, biobased feedstocks and reusable base chemicals from chemical recyclers can be used as raw materials which are sold to the base chemical producer. These feedstocks flow through the value chain ultimately to the end user. After which at the end of life, physical recycling, chemical recycling, or composting can bring these valuable materials back into the value chain to be used again.
FIGURE 1 Circularity in the coatings industry.
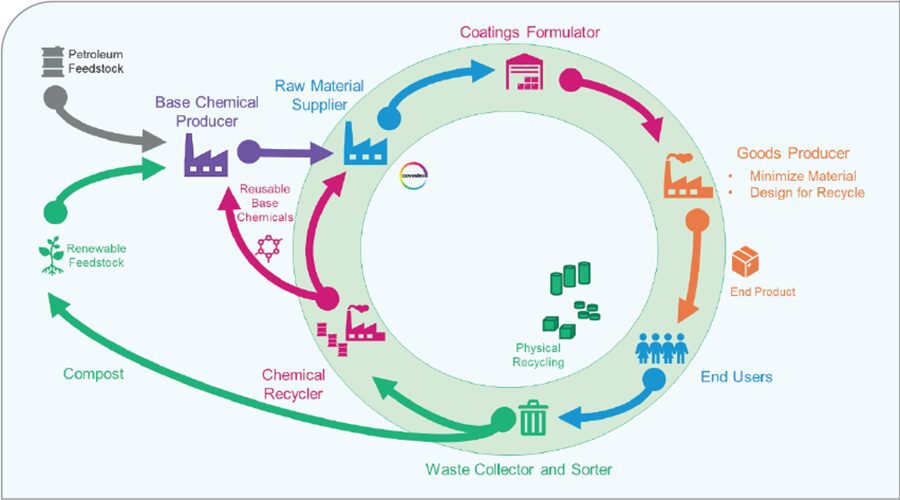
This article focuses on three areas: alternative raw materials, such as renewable feedstocks and mass balance feedstocks; the use of energy in application and curing; and, finally, the use of joint solutions in reducing emissions by utilizing high-performance water-based solutions to reduce emissions of carbon-based solvents. These will enable a coatings formulator to develop products with more circularity and sustainability built into them. The largest impact a coatings formulator can make is in the reduction and dependency on fossil-based raw materials. By focusing on these three areas, the formulator will have a direct impact on the circularity potential of their formulation. This transition will have the largest impact in reducing the overall CO2 footprint caused by coatings and processes.
14C Verified Renewable Feedstocks
Renewable feedstocks are those that can be 14C verified for bio-content. This means that their original source of carbon was plant based and not fossil based. In the polyurethane industry, great strides have been made to produce some of the key starter materials from biomass. Nonedible sugars from corn starch, wood, and straw can be converted through a bio-catalysis with micro-organisms to become amine starter materials. Three key amines in the polyurethane coatings industry are aniline, pentamethylene diamine, and hexamethylene diamine.
Bio-aniline is a very versatile building block. Today, its fossil-based alternative is a key raw material in the production of diphenylmethylene diisocyanate (MDI). MDI currently is the world’s largest volume diisocyanate produced. It is used in applications from mattresses, bumper foam, adhesives, and even polyurethane coatings. Bio-aniline will improve the CO2 footprint of the aniline process and is based on non-edible food feedstocks. Steilemann says, “Being able to derive aniline from biomass is another key step towards making the chemical and plastics industries less dependent on fossil raw materials.”
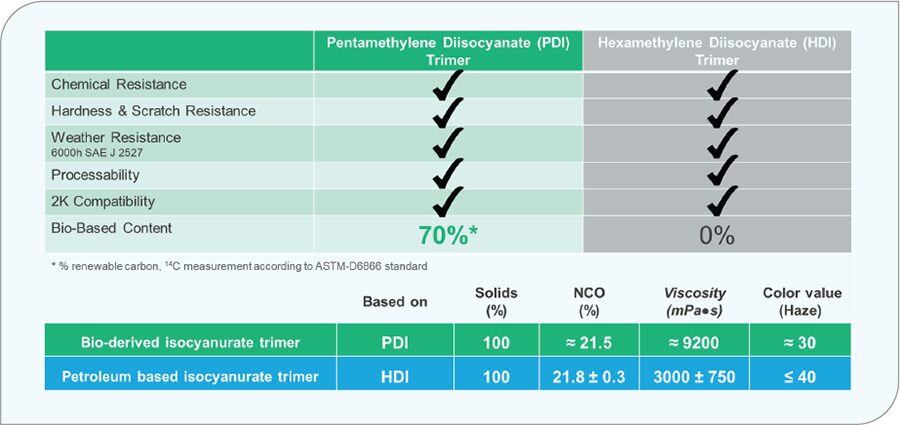
Pentamethylene diamine (PDA) is another biobased amine that is based on non-edible corn starch. When converted to the pentamethylene diisocyanate (PDI), it will contain 71% 14C verifiable carbon. As a polyisocyanate trimer, which is a polyisocyanate structure in coatings industry, it is very comparable to its fossil-based alternative, hexamethylene diisocyanate (HDI). Figure 2 illustrates the performance comparison of a fossil-based HDI trimer compared to the biobased PDI trimer. Here, it is demonstrated that there is no performance loss when switching to a biobased PDI trimer.
FIGURE 2 Performance comparison of PDI- and HDI-based trimers in a solventborne clearcoat.
Developments are also on the way to replace fossil-based hexamethylene diamine (HMDA) with a biobased alternative. This would allow for the production of biobased HDI. The current manufacturing process to make HMDA starts with the hydrogenation of adiponitrile that is produced from fossil-based butadiene. Through partnerships with Genomatica and Covestro, a biobased HMDA has been created. Polymers made from this biobased HDI have the benefit of 14C content along with being able to use current TSCA and DSL registrations because it is indistinguishable from the fossil-based HDI
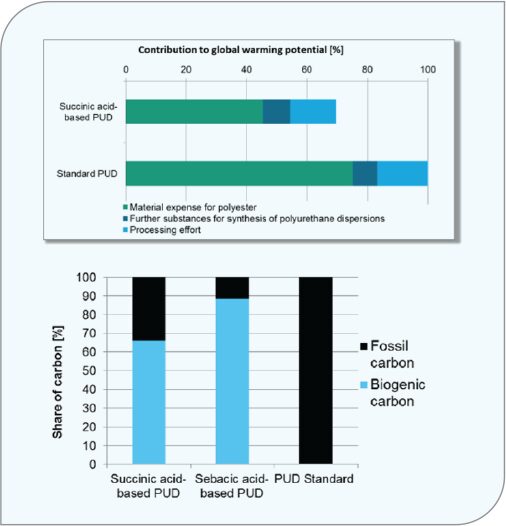
The other side of any polyurethane formulation is the resin. Typically, the largest contributor to the mass of a polyurethane, adipic acidbased polyesters are a key type of polyesters. Here, the 14C verifiable biobased succinic acid and sebacic acid have shown to be a good replacement for adipic acid in polyester manufacturing. When producing polyurethane dispersions with these biobased polyesters, a product with a renewable content as high as 65% is achievable. Figure 3 illustrates the reduction in global warming potential when switching to these biobased alternatives.
FIGURE 3 Global warming reductions comparing biobased polyesters to fossil-based polyesters.
When using these biobased building blocks, the formulator will no longer experience a reduction in physical properties previously expected with biobased raw materials. In some cases, such as oxidatively curable biobased building blocks, which are incorporated into a polyurethane dispersion, the resulting coating performs better than many of the competitors and can even compete with 2K options.
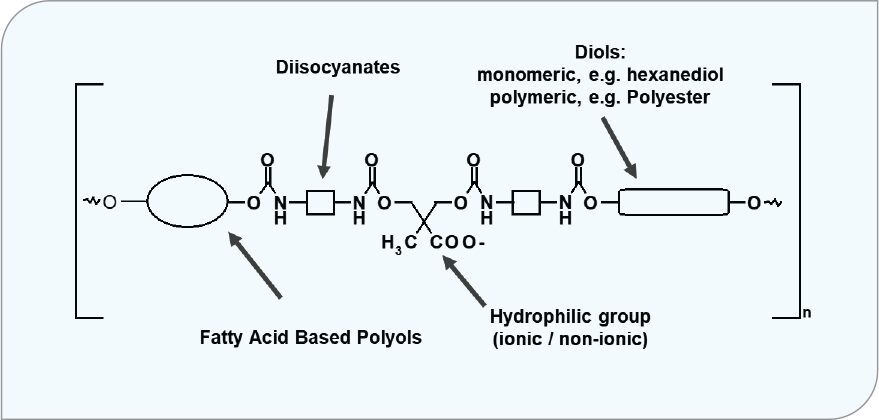
Figure 4 is a generic representation of how fatty acid biobased polyols are incorporated into oxidatively curable polyurethane dispersions.
FIGURE 4 Oxidative curable polyurethane dispersions having fatty acid-based polyols.
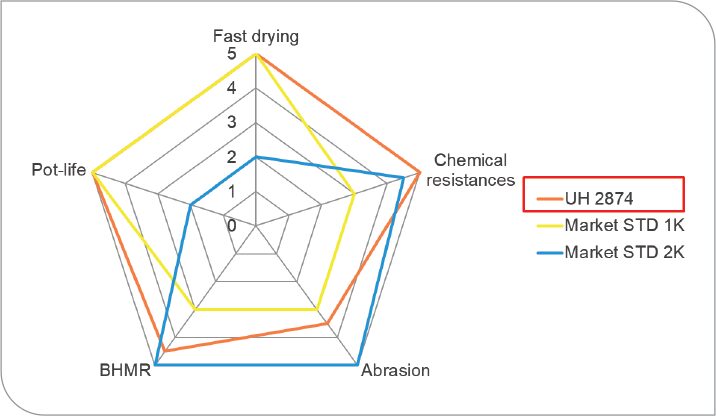
An example of is this biobased polyurethane dispersion is in a wood floor clear coating. With a 14C verifiable bio content of 49%, it has shown to be comparable to 2-component (2K) coatings on the market today. Figure 5 illustrates its performance against market standards in 1-component (1K) and 2K water-based wood floor coatings.
FIGURE 5: Comparison of oxidatively curable 1K clearcoat to market standards.
Certified Mass Balance Feedstocks
Mass balance is a certified manufacturing method that uses biobased and recycled feedstocks very early at the base chemical producer area of the circular value chain. ISCC+ (International Sustainability and Carbon Certification), an accreditation body, certifies each step along the value chain. Mass balanced materials are not 14C verifiable but are ISCC+ certified. Illustrated in the green circle in Figure 6 is where the certification process starts. Along each step of the value chain the ISCC+ certificate is passed along, and the next step is then verified until reaching the end user.
FIGURE 6 How mass balance is incorporated into circularity.
At the base chemical producer, renewable feedstocks, reusable base chemicals, and those from fossil-based feedstocks are blended in the process to make a base chemical. ISCC+ certifies and attributes a certain part of the production as biobased based upon the amount of biomass and recycled material in the process minus process scrap and expected yield. These certificates follow through each part of the value chain and ISCC+ will certify each step along the way. The role of mass balance is to accelerate the use of biomass and recycled materials without the need for new manufacturing facilities, new manufacturing processes, regulatory listings, and even final product validations. This process will reduce fossil resource consumption, waste streams, and environmental footprint. Mass balanced materials can be substituted for purely fossil-based ones without the need for reformulation or performance recertifications.
The list of available mass-balanced raw materials and volumes are growing every day. Many of those common raw materials for polyurethanes are now available. Isocyanate monomers such as MDI and TDI and aliphatic amines such as HMDA and IPDA are available today. Polyester building blocks, such as adipic acid, butanediol, hexanediol, neopentyl glycol, and trimethylolpropane, are available as well as solvents such as ethyl acetate, butyl acetate, and solvent naphtha. Incorporating these building blocks into product development help all of us reach our circularity goals.
Use of Energy
The use of energy is a key part in reducing the use of fossilbased materials. Here, the choice of a coating technology can play a crucial role. This can sometimes be a challenge for the coatings formulator because they are often constrained by a particular production line. A coating must be designed to work within this production line without the need for new construction or redesign. Performance is also key. End users are not willing to sacrifice performance to gain circularity benefits. The formulator must deliver on all three. For production lines that are designed to be baked, end users often wish to reduce their energy cost and increase efficiency. For these applications, thermolatent hardeners and polyaspartic coatings are two technologies that will do just that.
A thermolatent hardener is an HDI-based polyisocyanate trimer that will have a similar pot life to that of an uncatalyzed (no dibutyltin dilaurate) 2K polyurethane formulation.
Figure 7 illustrates three 2K solvent-based polyurethane coating formulations. Standard is uncatalyzed, catalyzed is a high-catalyst amount designed for fast oven cure, and the thermolatent is in blue. As shown, after mixing at room temperature, the thermolatent hardener behaves much like the uncatalyzed formulation. Once in the oven, the thermolatent formulation behaves much like the highly catalyzed one offering a triggered cure. Figure 8 shows an actual coating line for a plastic bumper.
FIGURE 7 The “Snap Cure” characteristics of a thermolatent hardener.
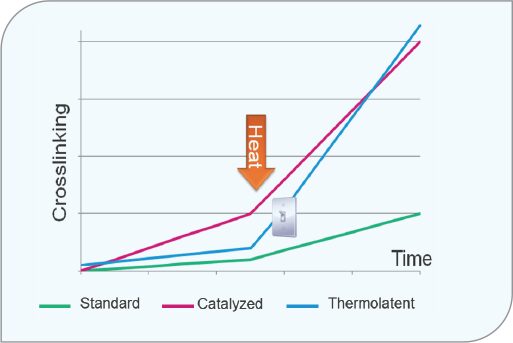
FIGURE 8 Typical real-life bumper coating line.
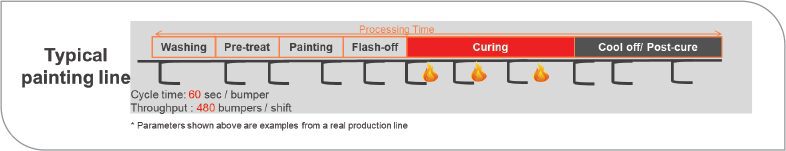
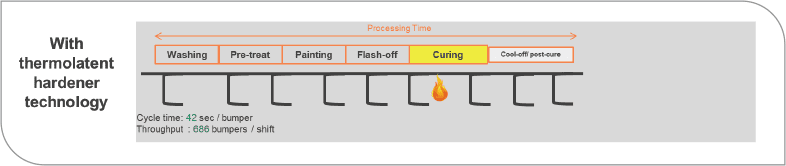
For the bumper coating example, an uncatalyzed coating is used. This is because it will give the best surface finish. It requires three baking zones and a significant post cure. This is because properties are not yet fully developed immediately out of the oven. Stacking and packing will usually occur sometime later. The highly catalyzed formulation is not shown as it did not have the appearance qualities required for the bumper. As illustrated in Figure 9, using the thermolatent hardener reduced the curing zones by two. This allows for faster stacking and packing of bumpers without damage. Energy reductions due to ovens were more than 65%. In this example, the thermolatent hardener reduced the cycle as well. Cycle time per bumper was reduced and throughput was increased by 30%.
FIGURE 9 Less energy and greater throughput possible with thermolatent hardener.
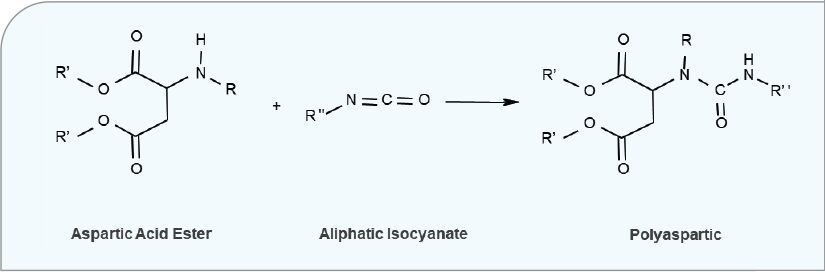
Polyaspartic coatings are another type of fast cure coating that does not require any heating for curing and can be applied using conventional 2K polyurethane spray equipment. Polyaspartic coatings are also known for being lower in VOC than other solvent-based polyurethanes. A polyaspartic is the reaction of an aspartic acid ester, a type of hindered secondary amine, with an aliphatic polyisocyanate. (See Figure 10.) The most common crosslinker used is a low-viscosity, HDIbased polyisocyanate trimer.
FIGURE 10 The polyaspartic reaction scheme.
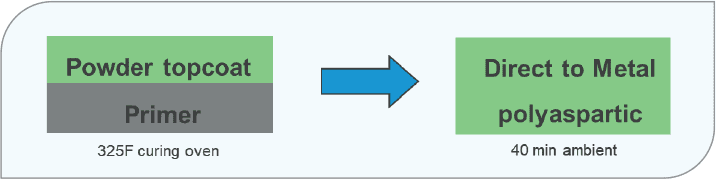
Polyaspartic coatings are known for improving coating efficiency. In many applications, polyaspartics can be applied directly to sand blasted steel in one coat with enough thickness to pass C3 corrosion protection. The elimination of a layer not only improves efficiency but also associated VOCs from the primer layer (Figure 11).
FIGURE 11 reduced numbers of layers and elimination of baking contribute to efficiency improvements.
This reaction is not accelerated by baking. Ambient atmospheric moisture is the catalyst for this reaction. The combination of physical attributes and curing needs were crucial in a light pole manufacturer being able to move from a baked 2-layer system to the single coat, ambient-cure polyaspartic. Because the process allowed for the curing ovens to be turned off, this manufacturer was able to save 75% of its energy cost.
The 40-minute ambient cure represented a 70% reduction in the coating process time per pole. Also, because polyaspartic can be electrostatically applied, the manufacturer also saw a 75% reduction in overspray.
Reducing Carbon through Reducing Emissions
Moving from solvent-based coatings to water-based coatings is a really good way to reduce the overall amount of carbon used in the coating process. In 2000, Bayer Corp. (now Covestro) won the Presidential Green Chemistry Challenge for the invention of 2K waterbased polyurethane coatings.5 The crucial innovation in this invention was the development of polyisocyanates that could disperse in water without the use of external surfactants.6,7
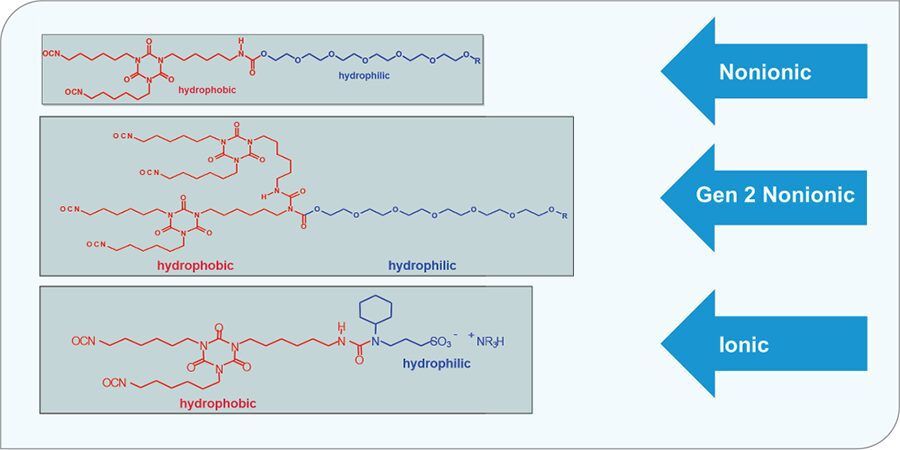
Figure 12 illustrates the three types of modified polyisocyanates most common today in 2K water-based polyurethanes. Nonionic types can be a prepolymer or a higher functional prepolymer, such as an allophanate-modified prepolymer. Ionically modified polyisocyanate are powerful in making emulsions and offer the highest level of hardness and chemical resistances. Typical co-reactants are hydroxy functional urethane dispersions or acrylic emulsions.
FIGURE 12 The three types of modified polyisocyanates used in 2K water-based polyurethanes. 40 PAINT.
Since 2000, these materials have been very successful in producing water-based polyurethane coatings with physical properties that rival traditional solvent-based ones. Successful market applications include concrete coatings, wood floor coatings, automotive plastic, primer coatings, and weatherable external metal coatings. Key features for these markets are 2K performance with organic solvent reductions of up to 99%.
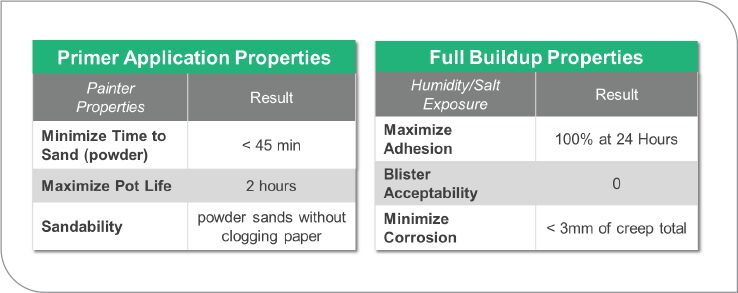
With the potential loss of Oxol 100 as an exempt solvent, there will be an increased need for 2K water-based coating formulations. Primer surfacers are one of those key application areas that rely heavily on exempt solvents. When Oxol 100 is delisted as an exempt solvent, formulations that now report a VOC of 2.1 lb./gal will increase to 3.5 lb./gal because Oxol 100 will again be included as part of the VOC calculation— and 2K water-based polyurethane will looked to as a solution. Today, formulations have been developed that hit the key performance parameters of a primer surfacer (Figure 13) and are able to do this at 1.75 lb./gal VOC without any exempt solvents being used.
FIGURE 13 The physical properties of a 2K water-based primer surfacer.
Another successful application for water-based polyurethane has been in the replacement of solvent-based rubber for temporary peelable coatings applications (Figure 14). In the automotive industry, the use of temporary coatings has been growing in aftermarket customization and protection. In this application the current solvent-based market standard has a VOC in the order of 5 lb./gal with 8 to 12 application coats needed to achieve a peelable coating. By switching to this 1K polyurethane dispersion, the use of organic solvent was reduced by 96% to a 0.2 lb./gal level. Performance benefits were also seen.
FIGURE 14 Comparison of oxidatively curing polyurethane dispersion to SBR peelable coatings.
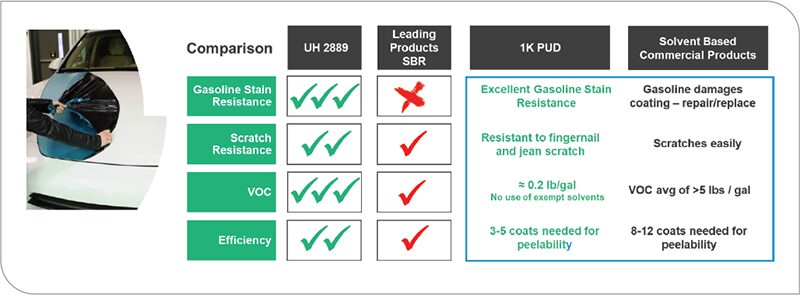
The market is also calling for another key attribute: to reduce coating layers. By using the 1K water-based polyurethane, the number of coating layers is now 3 to 5 layers. This is a reduction of about 60% and an improvement in efficiency. Gasoline resistance was another key attribute that the 1K waterbased polyurethane has that the market-leading solvated rubber product does not have.
Conclusion
It can be challenging for coatings formulators to incorporate sustainability and circularity into their product development. However, through using alternative materials, designing for less energy use, and working together, we can achieve the goal of moving from linear to circular value chains. We can reduce carbon usage, use waste as a valuable feedstock, and reduce our CO2 generation associated with making and applying high-performance coatings. As more and more sustainable and circular chemical streams come online, the future of sustainable and circular product development will become commonplace.
is the business development manager at Covestro. Email: mike.jefferies@covestro.com.
