By Gary E. Spilman, Mike Christy, and Brian Comstock-Reid, Resinate Materials Group, Inc.
Environmental, health, and safety concerns continue to drive awareness and interest toward improving the sustainability of materials used in the coatings industry.1 This is a subset of the larger movement toward becoming better stewards of global resources across all industries and working toward building a circular economy. To that end, this article discusses a nonpetroleum, non-bio resource for producing high performance industrial coatings resins. Alongside, and in harmony with current biobased efforts, Resinate has introduced commercial polyols that are designed to contain high levels of recycled materials. Previous presentations and publications2 introduced some of the descendants from recycled PET. Resinate now introduces multi-functional materials as base resins or blend additives, which are based on polycarbonate streams. Ultimately, these novel polyols provide a green chemistry alternative to heavy metal corrosion pigments, are nontoxic, can be added during the letdown stage of coating production, and can enhance other coating performance properties. This research compares performance in two separate formulated coating systems, and demonstrates the utility of these materials for metal protective coatings.
Introduction
Efforts have been underway to replace conventional petroleum-based feedstocks with newer biobased versions of the same materials. Although these biobased materials have provided feedstock options that are more sustainable than fossil petroleum alternatives, use of recycled content has remained relatively underutilized for high performance coating applications. To that end, we have tapped a nonpetroleum, non-bio resource for producing high performance industrial coatings resins. Along with current biobased efforts, commercial polyols have been introduced that were designed to contain high levels of recycled materials. These recycled materials that are normally destined for the landfill or incinerator can alternatively be upcycled and reprocessed into protective coatings that otherwise might incorporate virgin petroleum products.
The ability to reprocess thermoplastic materials mechanically and chemically opens many doors for reducing waste volume. The digestion of high relative molecular weight (Mr )3 polyesters is not a new concept.4 There have been many documented efforts, both past and present, in the reuse and repurpose of both industrial and consumer waste polyesters.5 The ability to melt process bulk thermoplastics back into useful forms such as pellet or flake for incorporation into new finished goods is a very convenient output for the collection, accumulation, and separation of such materials. According to the Association of Plastics Recyclers (APR), while the collection of HDPE bottles in 2014 rose to a rate of 31%, totaling nearly 1.1 billion pounds in the United States,6 the total weight of postconsumer polyethylene terephthalate (PET) bottles collected for recycling in the United States in 2014 was 1.8 billion pounds.7 The most widely recycled plastic is PET,8 a major component of many common consumer products including water and soda bottles, textiles, carpeting, auto interiors, and many packaging products.
Other materials, such as poly(bisphenol A carbonate), or PBAC, polyethylene, polyvinyl chloride, polypropylene, and polystyrene, can all be reprocessed through mechanical means such as crushing, shredding, chopping, etc., and can also be ultimately reprocessed in the melt into similar molded objects from which they came. Although they all have this in common, only the condensation materials, PET and PBAC, have a direct route to chemical breakdown due to the ester or carbonate linkage that holds them together, allowing them to be upcycled into higher-value, longer-life applications. Other materials are much less suited to this type of breakdown due to their chemical constitution, having an all-carbon backbone with no alternative functionality for targeting chemical attack. This makes them less prone to environmental breakdown as well. To utilize plastics recycle streams and leverage their chemical heritage, this chemical breakdown access is needed as a way to redesign the bulk properties of the materials. This is necessary to bring them into a useful range appropriate for the final application. For coatings, this mainly targets the glass transition temperature (Tg ) and the viscosity of the material. The chemical breakdown method is a gateway to designing lower Mr materials from their higher Mr predecessors, while providing building blocks for further optimization.
As previously mentioned, polyester has been taken through these modification steps; in many cases, all the way to its monomeric state, providing ethylene glycol and terephthalic acid.9 However, PBAC has had very little attention in this respect, especially for coatings applications. One of the major limitations with PBAC as a feedstock is its tendency to liberate bisphenol A (BPA) at high process temperatures, if proper catalyst is used, and especially when water is available. We have looked into this process, and have designed products that have excellent properties for coatings. These are produced through the degradation of high Mr recycled PBAC and result in useful polyols for metal protective films. They demonstrate good corrosion resistance and are helpful in many film properties that are desired for metal protection. The introduction of intermediates based on recycled PBAC has provided new alternatives to heavy metal anticorrosion pigments used in films for environmental barrier protection.
Recycled Material Feedstocks
The digestion of recycled PET can be accomplished through glycolysis at elevated temperature, with appropriate glycol/rPET ratios chosen for the desired oligomer distributions.10 Once this low Mr intermediate is established, the subsequent buildup using polyfunctional monomers can then reproduce desired Mr resins containing variable blocks of PET oligomers. The polyfunctional building blocks are mainly chosen from polycarboxylic acids and esters, vegetable oils, polyisocyanates, or other materials capable of reacting with hydroxyl groups. Most often, to achieve the highest total green content of the final material, selections from available biorenewable feedstocks are chosen. The combined total of both recycled content along with biorenewable content has been used to define the total “green” content of a material, and reflects the material’s total sustainability.
To produce hybrid systems from both polyester and polycarbonate recycle streams, an attempt was made to initially co-digest simultaneously in the same reactor using the same glycol and temperature profile, followed by the second stage molecular weight rebuild (with concomitant water evolution). This method was found to be the most efficient; however, it also produced the highest level of undesired color, as well as the highest level of free BPA from the polycarbonate. Various attempts and strategies followed this work, including modifications of:
- temperature profiles;
- reaction times;
- glycols used;
- catalyst choice and level;
- method of/point of introduction of PBAC; and
- various post-modification chemistry to scavenge BPA, including epoxy, acid, isocyanate, and other reactive functional groups.
A great deal of insight came from this work, and progress was made in generating newer and better intermediates for corrosion preventive coatings. The results ultimately led to some later products with very low BPA content and low viscosity. The common theme from all this work was discovering that every version of PBAC conversion product made by a variety of different processes ultimately demonstrated corrosion protection enhancement properties when evaluated under ASTM B117 salt spray conditions.
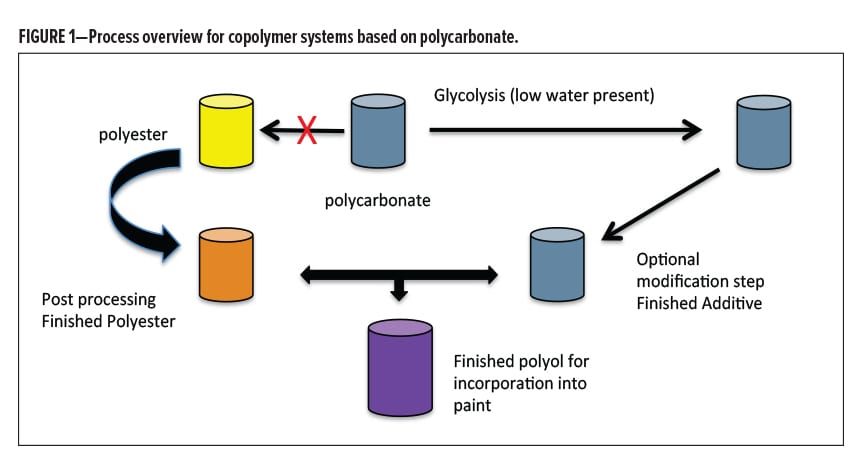 As the results became available from various process methods over time, the conclusions unambiguously directed us to avoid the co-digestion efficiency with subsequent rebuild step in a common reactor. Instead, separate digestions worked best, keeping the PBAC product isolated (and dry) with its own catalyst, temperature profile, glycol choice, glycol ratio (resulting in targeted Mr ) and cycle time, followed by a nonreactive blending step with a separate co-polyol (Figure 1). It was discovered that the free BPA generated by this process was an order of magnitude lower than previous methods, and the color development could be controlled by the catalyst choice and the temperature profile. A lack of exposure to condensation water coming from the Mr buildup step, as well as avoiding the use of secondary or tertiary hydroxyl functionality were both very helpful in limiting the production of BPA. Very reproducible blending polyols were made that performed consistently well in metal protection, along with reduced levels of undesired byproducts. The levels of free BPA in these systems were monitored, with good feedback on the chosen process variables. The separation of the two processes was very helpful in this overall effort. However, although very low free BPA resulted, by strict definition it did not quite fall below nonhazardous11 levels for reporting (< 0.1%).
As the results became available from various process methods over time, the conclusions unambiguously directed us to avoid the co-digestion efficiency with subsequent rebuild step in a common reactor. Instead, separate digestions worked best, keeping the PBAC product isolated (and dry) with its own catalyst, temperature profile, glycol choice, glycol ratio (resulting in targeted Mr ) and cycle time, followed by a nonreactive blending step with a separate co-polyol (Figure 1). It was discovered that the free BPA generated by this process was an order of magnitude lower than previous methods, and the color development could be controlled by the catalyst choice and the temperature profile. A lack of exposure to condensation water coming from the Mr buildup step, as well as avoiding the use of secondary or tertiary hydroxyl functionality were both very helpful in limiting the production of BPA. Very reproducible blending polyols were made that performed consistently well in metal protection, along with reduced levels of undesired byproducts. The levels of free BPA in these systems were monitored, with good feedback on the chosen process variables. The separation of the two processes was very helpful in this overall effort. However, although very low free BPA resulted, by strict definition it did not quite fall below nonhazardous11 levels for reporting (< 0.1%).
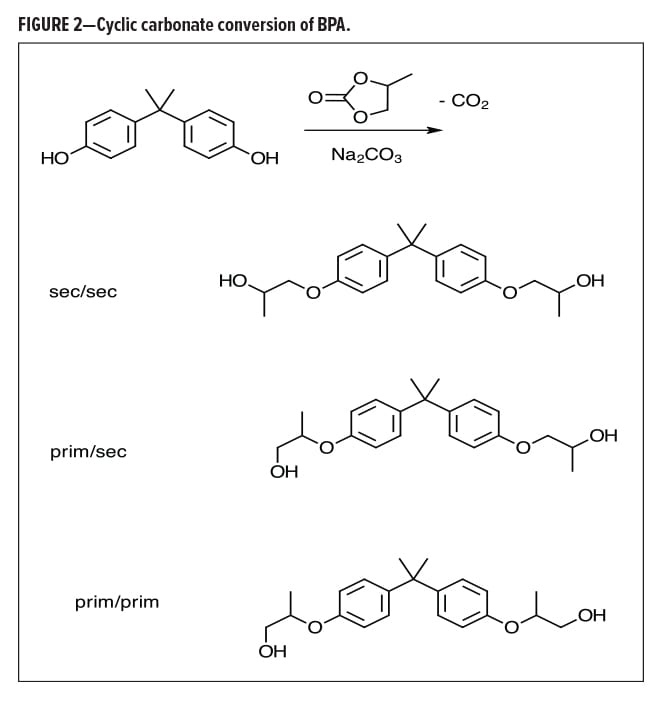
Almost a decade ago, Lin, et al.12 described the conversion of BPA to an alkoxylate or bis(alkoxylate) through the use of cyclic carbonates (Figure 2). Ethyl or propyl carbonate can nicely convert BPA to the corresponding ethoxylate or propoxylate, with high specificity for the phenol conversion in the presence of other hydroxyl species. This conversion was effective in still further reducing the BPA; however, process costs for extended cycle times along with reduced yields due to mass losses from CO2 are still being worked on.
Led by multiple successful polycarbonate intermediates, we ultimately developed a second-generation polyol product with nonhazardous (undetectable) BPA levels. It demonstrated excellent corrosion control performance, along with low viscosity, although it currently is not yet fully based on nonpetroleum ingredients. In this work, this newer corrosion control blending product Resinate® C2441 (Corrosion Control Polyol A) was compared to the earlier blending product based on recycled PBAC, Resinate PC1000-2.0 (Corrosion Control Polyol B).
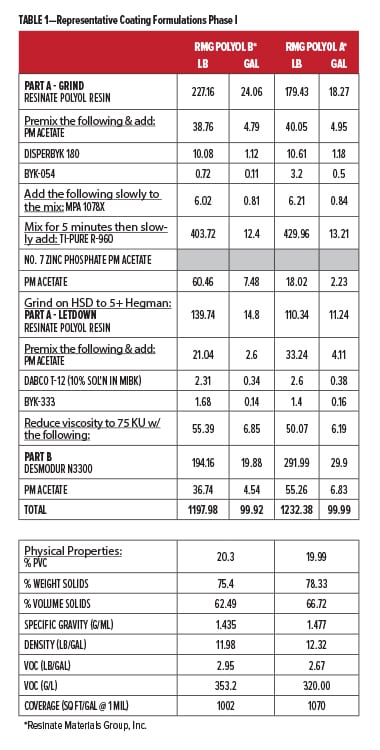
To validate the performance of both new corrosion control polyols, we collaborated with Stonebridge Coatings Laboratory, Inc. in Plymouth, MI. Stonebridge evaluated our new corrosion control polyols for performance enhancement of formulated coatings using our base polyesters. The film properties were evaluated, along with environmental testing for QUV-A and B117 salt spray performance (Table 2). In Phase I, we screened the newest polyol C2441 (Corrosion Control Polyol A) for possible inclusion in Phase II, where we then included our recycled PBAC oligomeric polyol PC1000-2.0 (Corrosion Control Polyol B) and some commonly used heavy metal additives for comparison. Phase I coatings were made at 20% pigment volume concentration (PVC)(Table 1), and for Phase II the PVC level was raised to 30% for primer applications (Table 3). Volatile organic content (VOC) calculations for the coatings were all around 3 lb/gal. For all experimental coatings based on Resinate C2901-04 (RMG Polyol A) polyester, a polyisocyanate based on hexamethylene diisocyanate (HDI) trimer was used at 0.8:1.00 NCO:OH due to the high average molar hydroxyl functionality (~4). A previous ladder study indicated optimal properties were observed at this NCO:OH ratio. In Phase II, the IMP1000-6.5 (Resinate Base Polyol) with functionality ~2 used a ratio of 1.05:1.00. Commercial coatings were prepared according to their manufacturer’s instructions at recommended ratios of parts A and B. For Phase I testing, film builds targeted 3.5 mil dry film, and for Phase II 3.0 mil dry film.
Coating Studies Phase I – Corrosion Control Polyol A Validation
Experimental
For this study, Stonebridge Labs performed all coating formulation, production, and application; also panel preparation, oven curing, environmental testing, and rating evaluations. Included were two commercial control benchmark (BM) coatings, a high-solids epoxy primer (BM-EP) and a DTM 2K urethane (BM-PUR). The commercial control coatings were fully formulated coatings for metal protection in different applications. The first comparison was between the epoxy primer and Resinate Materials Group (RMG) Polyol B polyester. The second experimental series was based on RMG Polyol A. Both RMG Polyols are considered semi-aromatic since they are composed of aromatic diacids and aliphatic glycols.13 RMG Polyol A is a DTM polyester, and we looked at this polyester alone, blended with Corrosion Control Polyol A (new corrosion control polyol) at two levels, and with Corrosion Control Polyol A in conjunction with a heavy metal corrosion inhibitor (zinc phosphate). This group was compared with the BM-PUR commercial coating (with zinc phosphate and UV stabilizer). The coatings were applied over aluminum and CRS via wire wound rod applicators, flashed for 30 min under ambient conditions, then baked for 30 min @ 130°C. They were allowed to age for seven days prior to testing and yielded DFTs of 3.5 +/- 0.5 mils. Multiple replicate panels were made. Prior work had indicated that 14 day ambient cure generated similar properties to the bake conditions used here. The panels were advanced to full cure much quicker, in the interest of efficiency. Both ambient and bake processed panels were examined in the second round.
Film Properties
After curing, all panels had greater than 6H pencil hardness by Wolff-Wilborn method (ASTM 3363). Crosshatch adhesion to untreated cold-rolled steel was 5B for both the epoxy and RMG Polyol B, only 0B for the commercial DTM urethane control, and the rest of the experimental coatings measured between 1B and 3B (ASTM D3359). The crosshatch grid provided a semi-quantitative method for rating adhesion from 0B (total coating removal) to 5B (no coating removal) using specified adhesive tape. Post-curing, all systems had >200 MEK double rubs (ASTM D4752) except for the commercial DTM urethane control, which had an average of 179.
QUV-A Performance
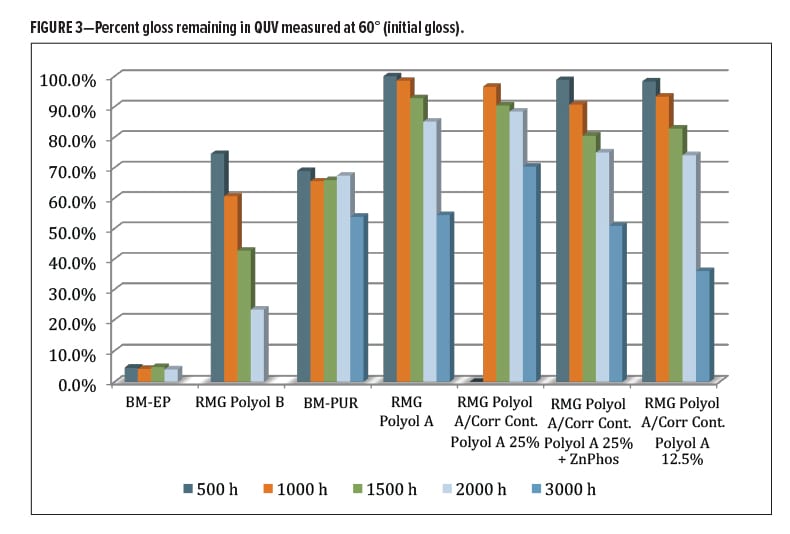
The QUV-A 340 nm (ASTM G154) performance of the coatings was evaluated over aluminum substrate for a total of 3000 h during which the gloss and color change were monitored. The percent original (60°) gloss remaining was less than 5% for the epoxy coating control (Figure 3). The RMG Polyol B was at 23% gloss at 2000 h, significantly better gloss retention than epoxy (BM-EP). The two primers were discontinued after 2000 h since their contribution to overall outdoor durability is highly dependent on what type and thickness of topcoat is used. The next best performer was the commercial control polyurethane (BM-PUR) coating, which stabilized at around 65% original gloss at 2000 h, then dropped further to around 53% at 3000 h. The rest of the coatings, all derived from the RMG Polyol A DTM polyester, had similar performance for gloss through about 2000 h. They ended up differentiated at 3000 h, where the 25% Corrosion Control Polyol A (without zinc phosphate) performed the best of the group holding 70% gloss. Overall, a good showing for the commercial RMG Polyol A series, most likely due to the polyester base resins with little effect from the blended Corrosion Control Polyol A.
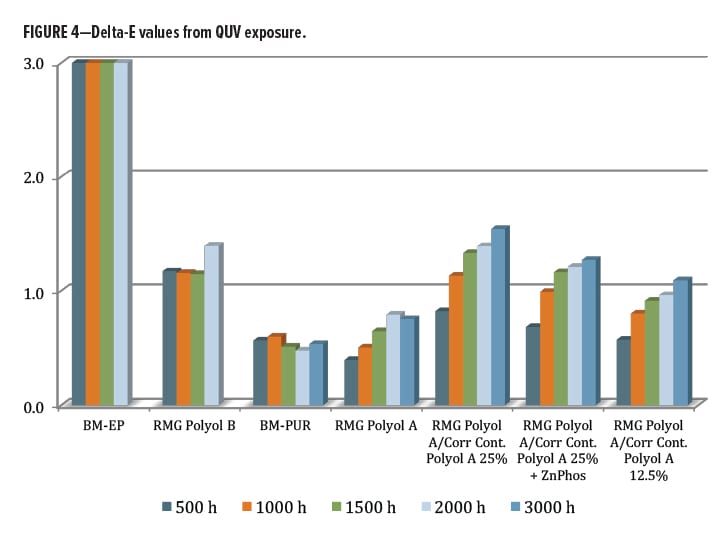
The Delta E value from QUV exposure (Figure 4) was also examined. Again, the worst performer was the commercial epoxy coating, with a change of almost 10 units after 500 h, but recovering to a final value of nearly 6 after 2000 h (chart is scaled to max value of 3). As the direct comparison, the RMG Polyol A aromatic primer polyol was significantly better with only a 1.4 unit shift after 2000 h. Both were discontinued beyond this point, as mentioned earlier. The commercial benchmark fully formulated urethane coating (BM-PUR) performed the best overall in this category at around 0.5 units color shift (with UV stabilizer). The RMG Polyol A experimental series contained no UV additive and had only a slightly higher Delta E of 0.75 for the base resin. This increased a bit more with the high and low levels of blended Corrosion Control Polyol A present. Unlike the gloss loss result, the color shift trended upward slightly with a higher level of Corrosion Control Polyol A. As a point of reference, color differences are normally seen at Delta E values of 1.0 or greater; however, in some cases the visual differences may not be perceived until the value is closer to 3.0.14 Overall, excellent performance in QUV-A exposure unstabilized was observed, with almost no UV effect from the blended Corrosion Control Polyol A.
Salt Spray Results
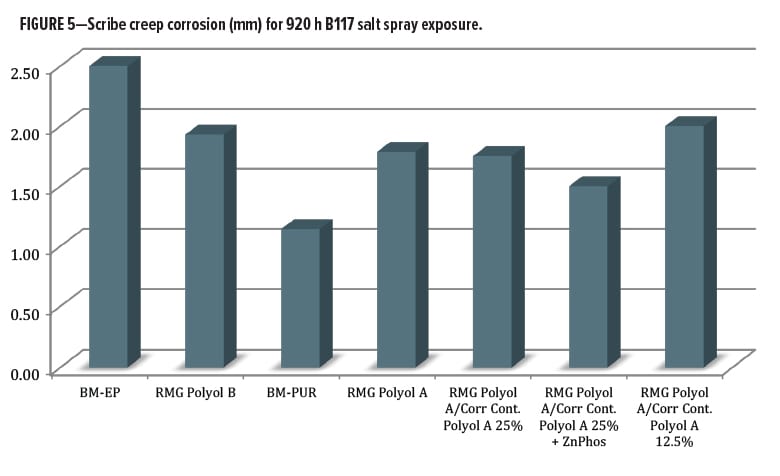
The salt spray testing was carried out for 920 h over untreated cold-rolled steel substrate. Data were averaged from multiple panel sets run simultaneously. Evaluations were made for blistering, field corrosion, scribe creep, and scribe rating. When the scribe creep was evaluated (Figure 5), values ranged from 1.0-2.5 mm, a narrow range. Surprisingly, the worst performance was from the benchmark epoxy coating (avg. 2.5 mm), compared to the RMG Polyol B primer at 1.9 mm. The best overall performance came from the commercial control DTM urethane. The Resinate Polyol A blend experimental series was closely clustered between the two commercial benchmark coatings. The differentiation between the control and the experimental polyols was minor at this level of exposure, as there was an average value of 1.1 mm for the commercial control urethane and 1.5 mm for the best experimental coating.
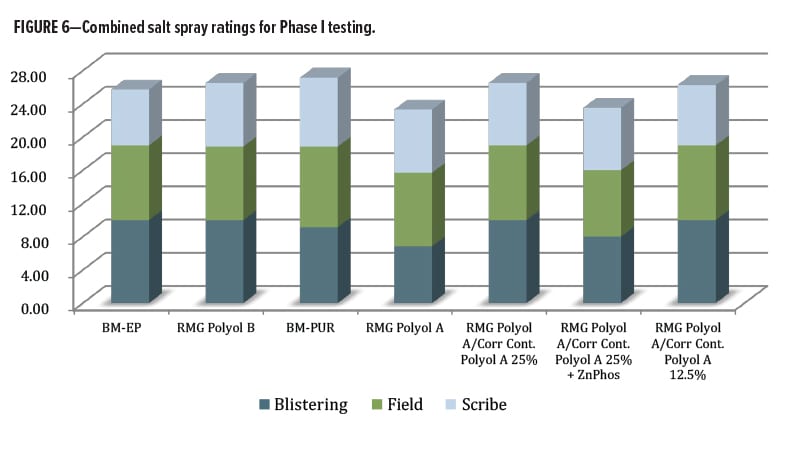
A broader perspective for rating the overall performance in salt spray was considered. The data from the ratings for blistering, field, and scribe were incorporated into a single metric—each rating had a maximum of 10 for best performance (no blistering, no field corrosion, less than 0.5 mm scribe creep) and these were combined into a single value (Figure 6). The commercial control urethane (containing zinc phosphate) only slightly outperformed both of the experimental Corrosion Control Polyol A blends, and by a slightly larger margin, the RMG Polyol A by itself and with zinc phosphate anticorrosion additive. It is unclear why the combination of Corrosion Control Polyol A with zinc phosphate did not outperform Corrosion Control Polyol A alone. However, the data indicate a positive effect from the Corrosion Control Polyol A material for use in metal protective coatings to replace heavy metal corrosion additives. The Corrosion Control Polyol A demonstrated it was worthy of further study by outperforming the zinc phosphate corrosion inhibitor additive. Next, the second round of testing with polycarbonate polyols began as well as some new benchmark control resins.
Phase II Coating Studies – Corrosion Control Polyol A vs Corrosion Control Polyol B vs Heavy Metals
Experimental
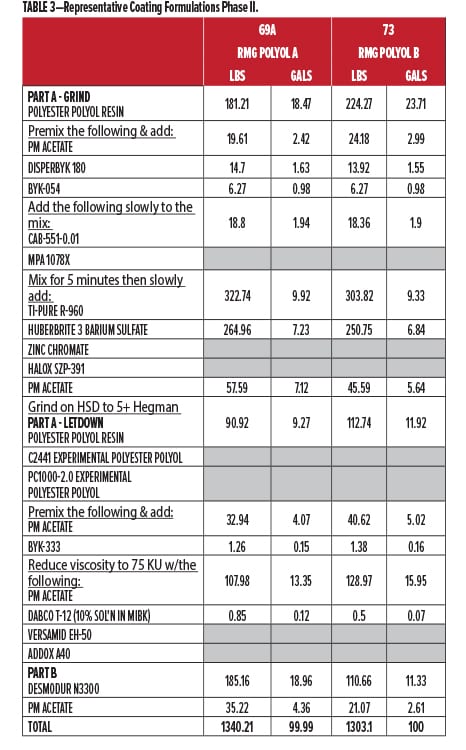
The second round of testing was done, as before, at Stonebridge Coatings Laboratory, Inc. Stonebridge conducted all of the coating formulation, production, panel preparation, oven curing, environmental testing, and ratings evaluation. The coatings were spray-applied over aluminum and CRS, flashed for 30 min under ambient conditions, then baked for 30 minutes @ 130°C. They were allowed to condition for 1-2 days prior to testing and yielded DFTs of 3.0 +/- 0.5 mils. A second set of panels was prepared, this time for ambient 2K curing over 14 days, for comparison of film properties to the oven baked set. The experimental base polyesters and corrosion control polyol resins were provided by Resinate for formulation into white coatings for testing.
In this round, new benchmark commercial controls were used; two commercial semi-aromatic polyols marketed for their excellent UV stability and high functionality (Control 1, 2) were chosen as direct competitors in the DTM market to RMG Polyol A. This series was prepared in a common formulation, applied to aluminum and steel panels, evaluated for film properties, and finally evaluated in QUV-A, as before.
In addition, a second series was added using a new Resinate polyester polyol base for interior/primer use (Base Polyol) as the main binder material. For this second set, the polyester was tested unmodified, with 5% zinc chromate, with 5% strontium zinc phosphate (SZP), with 20% Corrosion Control Polyol A, and with the newest recycled polycarbonate polyol, 20% Corrosion Control Polyol B. All materials were cured with commercial HDI trimer at 1.05:1.00 NCO:OH index.
Film Properties
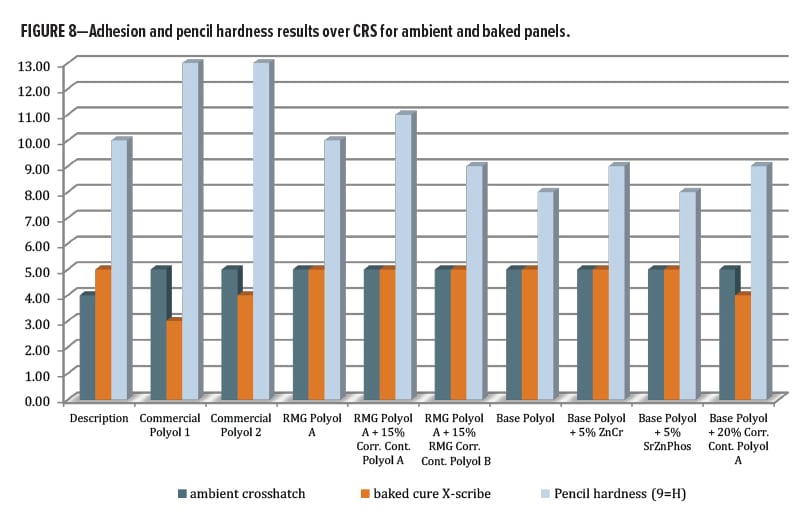
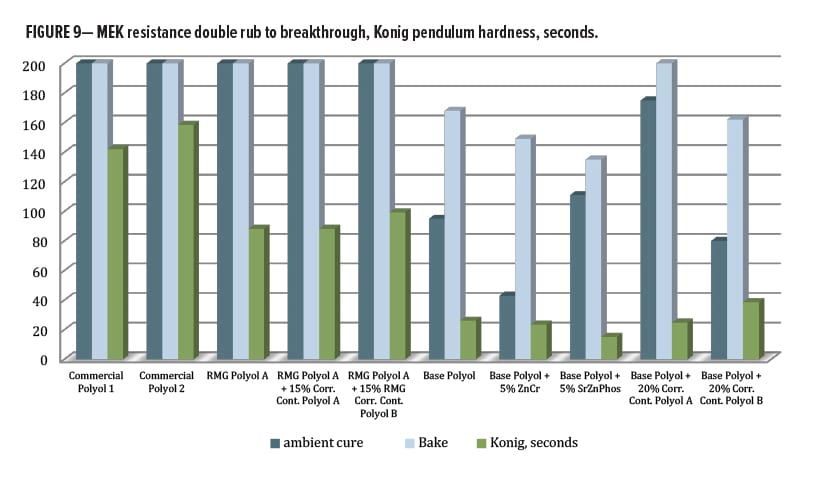
After oven curing at 130°C for 30 min, all panels had 5H-6H pencil hardness by Wolff-Wilborn method (ASTM 3363). Adhesion to untreated cold-rolled steel (CRS) was performed, this time by X-Cut (ASTM D3359) since the crosshatch tool could not fully cut through the coating film to the substrate. All tests were done in duplicate and measured 4A–5A for all, except Control 2, which averaged only 3A (Figure 8). Post-baking, both of the control polyols and all the systems based on RMG Polyol A had >200 MEK double rubs (ASTM D4752). For the series including base polyol IMP1000-6.5, the MEK values averaged between 140–160 except for the Corrosion Control Polyol A blended sample, which improved double rubs to an average of 195.
The second set of panels was allowed to cure under ambient conditions for 14 days, before film properties were analyzed. The hardest films by pencil scratch were the RMG Polyol A and Control 2. These films both measured 5H pencil hardness after ambient cure. The RMG Polyol A matched up nicely with the commercial controls for hardness. The primer series trended softer in general after ambient cure.
Adhesion testing for the ambient cured panels could be obtained using a crosshatch tool. Every composition in this study gave a 5B result (Figure 8) except for Control 1, which was 4B. MEK resistance (Figure 9) for the ambient cured set was mostly similar to that for the baked panels except for a large drop with the ZnCr panels. Both control polyols were able to achieve over 200 double rubs without breakthrough for both ambient and baked cure, as was the RMG Polyol A and its two blends. The series based on IMP1000-6.5 had lower hydroxyl functionality per chain, which resulted in lower crosslink density, and so all had slightly lower MEK resistance. The base polyester alone provided 95/168 double rubs for ambient/bake, respectively. The zinc chromate addition dropped both values to 43/149, while SZP addition gave 111/135. The addition of the corrosion inhibitive pigments seemed to reduce the chemical resistance by some mechanism. Interestingly, for the Base Polyol (Resinate IMP1000-6.5) series, blending Corrosion Control Polyol B dropped MEK double rubs very slightly to 80/162, while blending Corrosion Control Polyol A increased them to 175/200, an excellent result.
QUV-A Performance
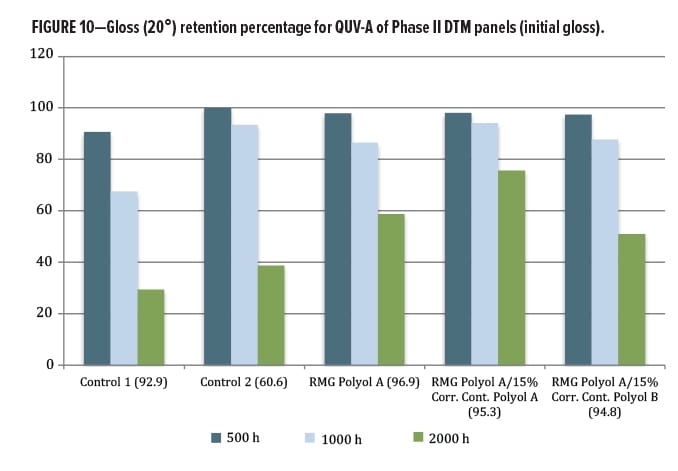
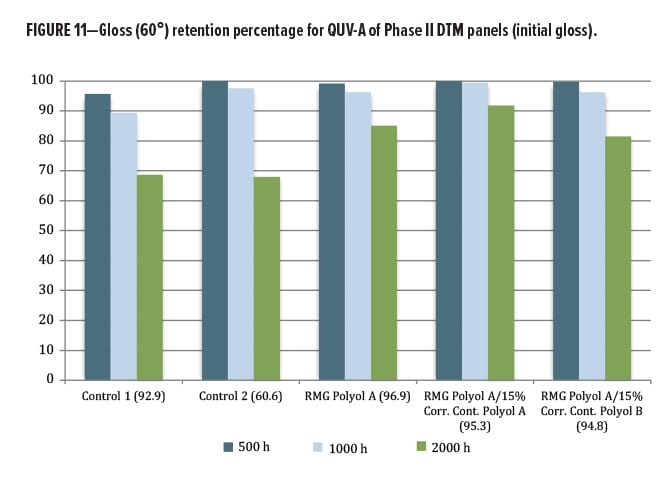
The QUV exposure focused on the DTM/topcoat polyol series. The other materials were designed for a primer function, and so were not exposed in this round. Since the results for this second phase are relatively new, only 2000 h of data from QUV-A exposure were available. Again, gloss loss (Figures 10, 11) and color change by Delta-E (Figure 12) were examined. Both commercial control polyesters used in this study were marketed as having excellent lightfastness, gloss retention, and weather stability, yet did not perform as well as the RMG Polyol A, where addition of Corrosion Control Polyol A actually improved gloss retention slightly over the unblended base. A very slight change was observed with addition of the Corrosion Control Polyol B, consistent with excellent outdoor performance as a DTM coating.
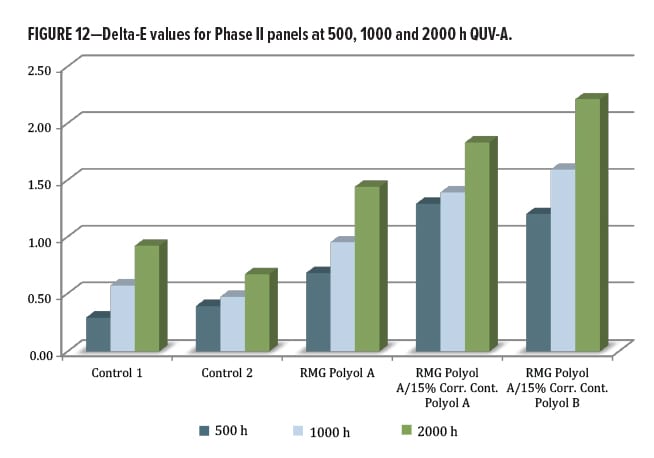
The delta-E values were low for the two control polyols, between 0.5–1.0 units at 2000 h exposure (Figure 12). The RMG Polyol A was below 1.0 unit at 1000 h, and slightly above 1 unit at 2000 h. When containing corrosion control polyols, the RMG Polyol A product is slightly more susceptible to yellowing, but again was without any UV stabilization.
Salt Spray Results
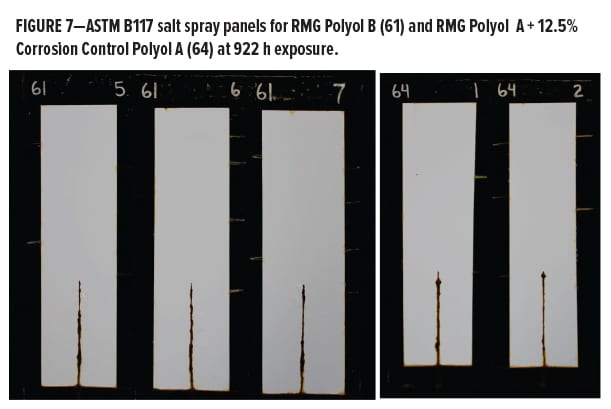
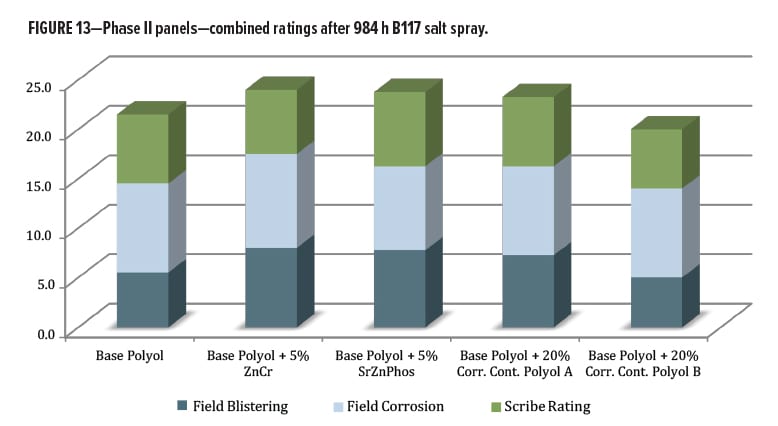
The salt spray testing in Phase II was carried out for 984 h over untreated cold-rolled steel panels, and focused on the primer series. Evaluations were initially made for blistering, field corrosion, and scribe creep (Figure 13). The measured scribe creep values ranged from 0–4 mm at 1000 h, and most were clustered between 1.0–2.5 mm, so there was small differentiation for this level of exposure. Field corrosion ratings for all panels only ranged between 8.0 and 9.5; there was again not much differentiation. The information gathered by this metric only takes into account the visible changes on each panel as they appeared after removal from the corrosion chamber and inspection. This initial collection of data told only part of the story about the total effects of the B117 exposure environment on the scribed panels.
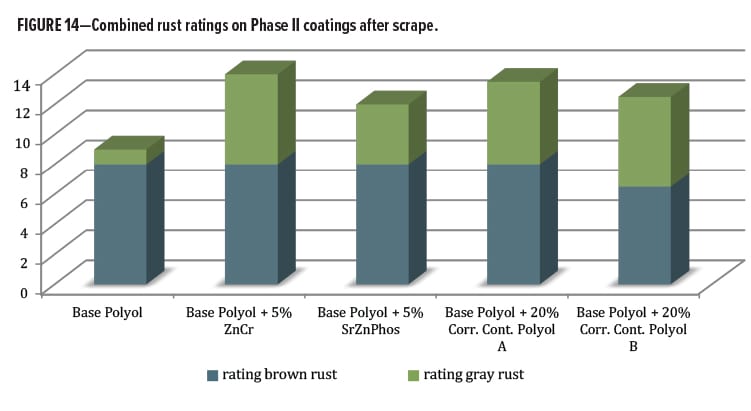
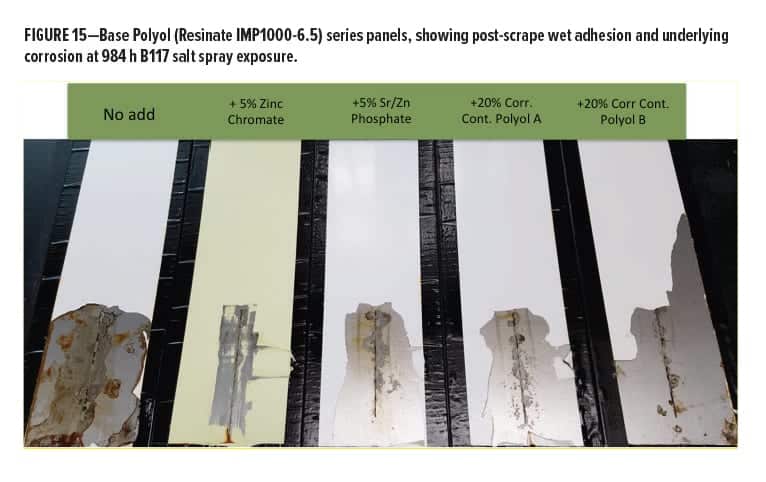
Additional information was obtained by rating field corrosion near the scribed region after scraping. This process removed coating film that was not well adhered and had allowed varying degrees of corrosion activity to occur beneath the film. This corrosion activity became exposed when the coating was removed by scraping along the scribe with a metal blade. Data are shown in Figure 14. Some differentiation was noticed when the gray and brown rust areas were rated and combined. The Base Polyol coating had the lowest performance ratings, followed by the rest of the series containing various anticorrosion additives. They all had a positive effect in this measurement category vs the polyol alone. The best of the entire group contained zinc chromate, followed closely by the Corrosion Control Polyol A blend. Below this level were the Corrosion Control Polyol B blend followed by the SZP pigment.
It was later decided after discussion with Stonebridge to perform an additional perpendicular scrape from the scribe to the right edge of the panel to remove excess delaminated coating laterally from the scribe as well. It is interesting to note here that although coating films were penetrated by moisture resulting in wet adhesion failure, there was still a significant difference in corrosion activity between the unmodified blank polyester and the coatings containing the Corrosion Control Polyol A or B. Beneath the coating films was some evidence of minor corrosion events, similar to the observed effects of cathodic disbondment or cathodic delamination and blistering of the coating film at the metal surface under cathodic protection, along with some degree of passivation. So, although the adhesion had some moderate failure at this point, there was still varying degrees of metal protection visible (Figure 15). Possibly some residual barrier protection remained beneath the delamination and the issue related to adhesion can be addressed separately. More work is planned in this area. A recent paper given at the Waterborne Symposium15 described observations from styrenated-acrylic systems that had inversely correlated aluminum crosshatch adhesion with good corrosion resistance, although further study supported a stronger correlation of corrosion resistance with low frequency impedance. Whether several simultaneous mechanisms contributing to corrosion can be independently addressed remains to be determined.
As Figure 15 unambiguously shows, the Base Polyol containing zinc chromate corrosion inhibitor was the clear frontrunner in this study. This additive is very well known for its excellent anticorrosion properties, although chromates have become less favorable from an environmental, health, and safety perspective. Additionally, it was noted that “although zinc phosphate is typically preferred over older traditional highly toxic lead and chromium based anticorrosive agents, it still poses environmental health problems. Zinc phosphate has been shown to be toxic to aquatic life.”16
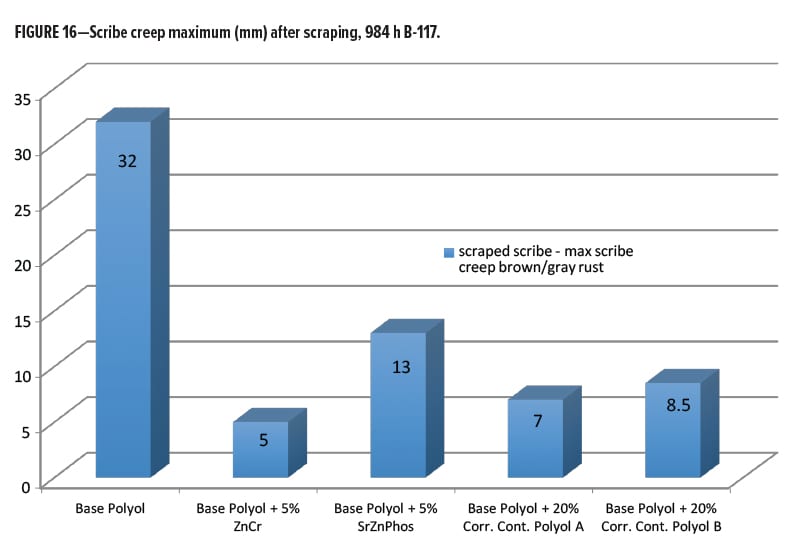
The next best performance after chromate came from the Corrosion Control Polyol A (Resinate C2441), and Corrosion Control Polyol B, where there was little significant differentiation. As a final metric for rating the performance after scraping the scribes, a plot of the maximum scribe creep corrosion measurement on each panel was made and is shown in Figure 16. Here we can see the worst performance came from the unmodified base polyester polyol, as expected. The best was the chromate modified, followed closely by the Corrosion Control Polyol A, and Corrosion Control Polyol B modified coatings. The SZP additive had almost twice the maximum scribe creep as the Resinate corrosion control polyols.
Summary
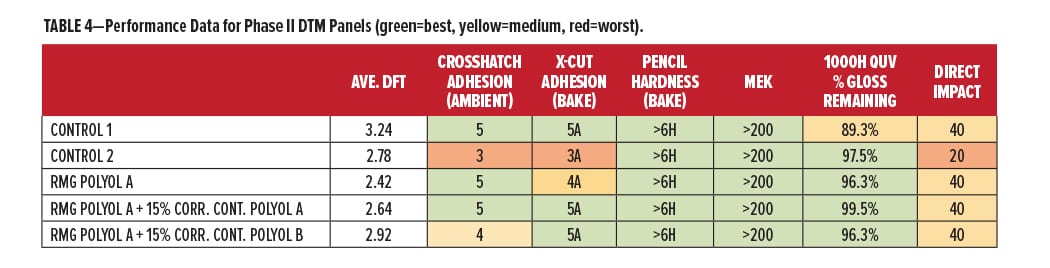
Further investigation was conducted in the Phase II evaluations using 30% PVC coatings. RMG Polyol A for DTM applications and RMG Polyol B for primer applications were evaluated with Corrosion Control Polyols. A summary of the performance properties for RMG Polyol A vs commercial control polyols is shown in Table 4. The green areas are performing at or above expected levels, yellow areas slightly below, and red areas need some improvement. RMG Polyol A with Corrosion Control Polyol A has the most green performance color on the table, to accompany its “green” content of recycle and renewable.
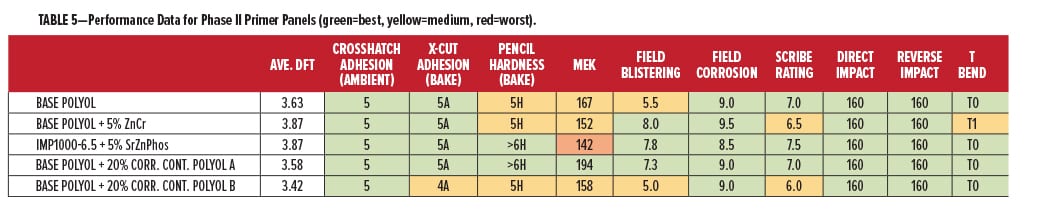
The overall film performance data for the primer compositions is shown in Table 5, presented in the same color format. The data in Table 5 are comparing the Base Polyol vs the four corrosion additive blends made from it. It is important to note that the corrosion control polyols are not used in combination with any other form of inorganic “sacrificial” or “passive” protection. If we combine the film performance and corrosion performance to rank overall protection for metal, we see that the overall best in this study was the Base Polyol, once again with 20% Corrosion Control Polyol A. Adhesion, hardness, chemical resistance, impact, flexibility, and corrosion performance were all within the highest category (green). The combined data also indicate some of the relative comparisons and tradeoffs in using heavy metal corrosion inhibitors, which performed very similar with some slight deficiencies in chemical resistance and flexibility. With the coating formulation results from this study, there are a number of good reasons for continuing exploration of this highly sustainable technology.
It was expected that the commercial corrosion inhibitive pigments would lead the performance here, but very similar or better overall protection from the polycarbonate derivatives Corrosion Control Polyol B and Corrosion Control Polyol A was observed. These polyols are performing on a par with some well-established corrosion control products, and will be further investigated for their performance advantages in sustainable coatings.
Conclusions
In a 20% PVC coating screening study:
— RMG Polyol A provided excellent DTM properties vs a commercial 2K polyurethane coating. It outperformed in QUV gloss retention; it delivered equivalent delta-E performance; and it provided similar B-117 salt spray ratings when using Corrosion Control Polyol A.
— Meanwhile, RMG Polyol B with 60% combined recycle and biorenewable content in a primer coating outperformed a commercial epoxy primer. It delivered both improved QUV gloss and delta-E; and outperformed in combined B-117 ratings for blistering, field, and scribe corrosion.
Subsequently, a 30% PVC coating study demonstrated the performance of Corrosion Control Polyol A. It outperformed strontium zinc phosphate overall in B-117 salt spray at 984 h in both unscraped and scraped scribe ratings; and the coating performance properties yielded improved adhesion, hardness, chemical resistance, flexibility, and impact.
Ultimately, these novel polyols provide a green chemistry replacement option for heavy metal corrosion pigments, are nontoxic, can be derived from recycled content, can be added during the letdown stage of coating production, and enhance coating performance properties. Research has revealed the next generation of sustainable corrosion prevention for the future of metal substrates.
Acknowledgments
The author wishes to acknowledge the entire Resinate Materials Group technical team, especially Adam Emerson, Matt Brown, Samantha Pilon, Crystal Simos, Michelle Samson, and Rick Tabor. Assistance with preparation of this paper, and the materials and testing required to develop the technology was due to their efforts. Additional assistance from Kris Weigal and Kristina Marsh in the preparation of the final manuscript is also gratefully acknowledged.
References
- a. Mash, T. “Sustainability in the Coatings Industry.” PCI Magazine [Online] 2015, https://www.pcimag.com/articles/100363-sustainability-in-the-coatings-industry (accessed Mar 2017). b. https://www.paint.org/about-our-industry/sustainability/
- a. Spilman, G., Tabor, R., Emerson, A., Brown, M., Christy, M., Hense, D., and Jackson, M., “Upcycling from Water Bottles to Protective Coatings: A New Approach with New Performance–Part I.” PCI Magazine (Nov. 2015). b. Spilman, G., Emerson, A., Christy, M., Fryz, G. “Upcycling from Water Bottles to Protective Coatings: A New Approach and a New Life – Part II.” PCI Magazine (Apr. 2016). c. Spilman, G., Emerson, A., Beatty, M., Brown, M., Christy, M., Kovsky, J., Rogers, K., Vrabel, E., and Tabor, R., “Creating Multifunctional Coatings Using Recycled Raw Material Streams,” CoatingsTech (June 2015). d. Spilman, G. and Emerson, A., “Recycled-Content Polyols for Specialty Polyurethane Coatings,” PCI Magazine (May 2015).
- Coghill, A.M. and Garson, L.R. (Eds.), The ACS Style Guide–Effective Communication of Scientific Information 3rd Ed., Oxford Univ. Press: Oxford, 2006; p 189. Note: preferred reference to the term molecular weight is Mr according to this source.
- a. Vaidya, U.R. and Nadkarni, V.M., “Polyester Polyols for Polyurethanes from PET Waste,” J. Appl. Polym. Sci., 35, 775-785 (1988). b. Vaidya, U.R. and Nadkarni, V.M., “Polyester Polyols from PET Waste: Effect of Glycol Type on Kinetics of Polyesterification,” J. Appl. Polym. Sci., 38, 1179-1190 (1991).
- a. Coelho, T.M., Castro, R., and Gobbo, J.A., “PET Containers in Brazil: Opportunities and Challenges of a Logistics Model for Post-consumer Waste Recycling,” Res., Con. and Recycling, 55 (3), 291-299 (2011).
b. Niaounakis, M. ,Biopolymers: Reuse, Recycling, and Disposal; Elsevier: Amsterdam, pp. 151-188, 2013. - “Plastic Bottle Recycling in the U.S. Tops 3 Billion Pounds for First Time,” The Association of Plastic Recyclers (APR) website, https://www.plasticsrecycling.org/news-and-media/424-november-5-2015-plastic-bottle-recycling-rate-report-release (accessed June 2017).
- Report on Postconsumer PET Container Recycling Activity in 2014, Oct. 2015, The Association of Postconsmer Plastic Recyclers and NAPCOR;
NAPCOR_2014RateReport_FINAL.pdf (accessed June 2017). - NAPCOR National Association for PET Container Resources, (accessed June 2017).
- a. Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, Gh; Rabie, A.M.; ElMetwally, A.E., “Greener Routes for Recycling of Polyethylene Terephthalate,” Egyptian J. Petroleum, 25 (1), 53-64 (2016).
b. Oku, A., Hu, L.-C., and Yamada, E., “Alkali Decomposition of Poly(ethylene terephthalate) with Sodium Hydroxide in Nonaqueous Ethylene Glycol: A Study on Recycling of Terephthalic Acid and Ethylene Glycol.” J. Appl. Polym. Sci., 63 (5), 595-601 (1997). - Patel, M.R., Patel, J.V., Mishra, D., and Sinha, V.K., J. Polym. Environ, 15, 97-105 (2007).
- Hazard Communication Hazard Classification Guidance for Manufacturers, Importers, and Employers; Occupational Safety and Health Administration, 2016. https://www.osha.gov/Publications/OSHA3844.pdf, [Online, accessed Mar 2017)
- Lin, C-H, Lin, H-Y, Liao, W-Z, and Dai, S., Green Chem., 9, 38-43 (2007).
- Polymer Properties Database, https://polymerdatabase.com/polymer%20classes/Polyester%20type.html, [Online, accessed Sept. 2017].
- Helsel, J.L.,” Avoiding Fading Paint,” Durability and Design, 45-51 (June 2014).
- Bulick, A.S., LeFever, C.R., Frazee, G.R., Jin, K., and Mellott, M.L., “The Impact of Film Properties on Corrosion Resistance in Waterborne Acrylics and Next Generation Low VOC Resin Development,” In The Waterborne Symposium, Proc. 44th Annual International Waterborne, High-Solids, and Powder Coatings Symp., New Orleans, LA, Feb. 19-24, 2017; Storey, R.F. and Rawlins, J.W. (Eds.), Lulu Press Inc., Raleigh, NC, 2017.
- Pasternack, G. “Zinc Phosphate Voted as Most Promising Anti-corrosive Pigment for Coating Protection,” SpecialChem / Jan. 2013. https://coatings.specialchem.com/tech-library/article/zinc-phosphate-voted-as-most-promising-anti-corrosive-
pigment-for-coating-protection, (Online, accessed July 2017).
CoatingsTech | Vol. 14, No. 10 | October 2017