 This column is a summary of the Analytical Series article, “Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy Part 1: Fundamentals of Electrochemical Impedance Spectroscopy,” by David Loveday, Pete Peterson, and Bob Rodgers of Gamry Instruments, published in the August 2004 issue of CoatingsTech. The complete paper is available here as part of our special collection, Analytical Series: From the Archives.
This column is a summary of the Analytical Series article, “Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy Part 1: Fundamentals of Electrochemical Impedance Spectroscopy,” by David Loveday, Pete Peterson, and Bob Rodgers of Gamry Instruments, published in the August 2004 issue of CoatingsTech. The complete paper is available here as part of our special collection, Analytical Series: From the Archives.
Electrochemical Impedance Spectroscopy (EIS) is a powerful technique that can be used to study the corrosion protection provided by coatings and the mechanism(s) when they fail. This information can assist scientists in assessing how well and how long a coating can protect the substrate, and how to improve its performance. Although this technique typically is used in the laboratory, instrumentation is available to assess performance in the field (e.g., railcars, bridges, etc.).
Corrosion is an electrochemical process of metal degradation, including painted metals, in which electrons move from one part of metal (anode) to another (cathode). With the movement of electrons, the process can be described as an electrical circuit, and the components within this system (i.e., coating, metal, electrolyte) act like and can be modeled as elements such as resistors and capacitors within a circuit, along with their associated parameters. In direct current (DC) systems, R (resistance) = V (voltage)/I (current). For alternating current (AC) systems, impedance, Z, is a circuit’s tendency to resist (or impede) alternating electrical current. The related relationship is Z = V/I. EIS can be used to measure these parameters. For example, EIS can be used to measure the impedance over a wide range of frequencies of a coating system exposed in a corrosive environment such as saltwater.
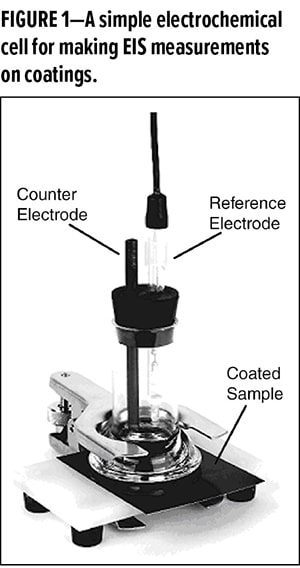
Figures 1 and 2 show an exposure cell and instrumentation used for EIS measurements. The cell is constructed in the same manner as used in electrochemical measurements, such as studying corrosion. A sample electrode (working electrode; i.e., coated metal), a reference electrode (e.g., saturated calomel), and a counter electrode (inert material; e.g., platinum) are immersed in an electrolyte solution (e.g., 5% NaCl, common for corrosion testing).
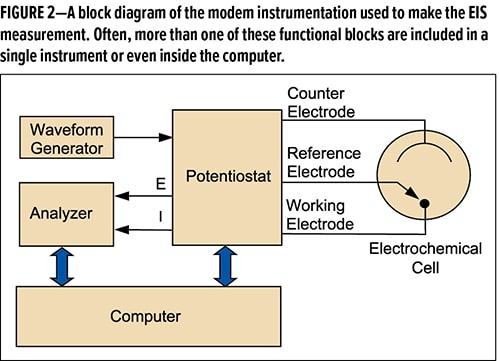
EIS instrumentation includes a waveform generator to produce sine waves and a potentiostat to control the potential. It controls the DC potential as well as the AC voltage. The instrumentation measures AC voltage, the current and the phase relationship between them over a range of frequencies. This data is used to calculate the impedance of the system. The instruments are connected to an electrochemical cell which contains the coated metal. A computer runs the experiment, which includes collecting and analyzing the data. EIS experiments can expose and measure the parameters at a specific point in time, but also measure the parameters as exposure time continues, thus allowing assessment of coating degradation and/or corrosion events over an extended exposure period. The sample can contain any metal/coating /electrolyte desired, preferably ones that represent real world conditions.
For example, studying a marine environment, a cell may consist of a steel alloy with a marine coating which is immersed in salt water. To study corrosion that may occur in a vehicle radiator, one may choose a copper alloy, a protective coating, and an antifreeze solution. Performing EIS studies over exposure time will provide information on any coating degradation and/or substrate corrosion, if any. This information will give valuable clues on the mechanisms occurring over time, which can then be addressed to improve performance.
The data collected includes the frequency of the AC waveform and either the magnitude and phase of the impedance at each frequency, or the real and imaginary components of the impedance. Modern equipment and software perform this task and assist in analyzing the results. Common output displays are Bode (Impedance and Phase Angle vs. Frequency) and Nyquist (Imaginary vs. Real Impedance) plots, which will be described in more detail in the next column on EIS. Once data has been collected, it is analyzed. The software can create an equivalent circuit model. This approach makes the analogy between the electrochemical cell (i.e., corrosion or lack thereof) and electrical circuit components such as resistors and capacitors. In short, this clearly indicates how good (or bad) the coating is performing. In greater detail, it provides information on how the coating may be failing.
In the October 2021 issue of CoatingsTech, Part 2 of this summary will focus on what EIS can tell us about coating performance and failure mechanisms.
CoatingsTech August 2021 | vol. 18, no. 8
