By Jessica Levin, Wenqin Wang, Stan Brownell, Tara Conley, Erica Frankel, John Rabasco, Deb Graves, and Adrian Ward,
Dow Chemical Company, USA
Increasing regulatory restrictions mean that there are limited preservation options currently available to the paint and coatings industry for both in-can and dry-film preservation. Experimental binders and thickeners that are more robust to microbial spoilage offer a potential solution and pass challenge testing even when formulated into waterborne paints.
Water-based products are susceptible to microbial contamination. Contamination can be introduced during a variety of stages in the product life cycle, including manufacturing and packaging of the products; “in can” during periods of storage, transportation, transfer, and usage; or on the dry film after application. Microbial susceptibility can cause product degradation, reduce product performance, or even induce hygiene and human health issues, which could result in a wide range of possible consequences, including product recall, customer complaints, reduced perception of product quality, production stoppage, etc. For these reasons, manufacturers add biocides to their waterborne products.
There are three aspects of coating preservation. The first is in-can preservation, which protects all liquid-state products with preservatives. The second aspect of preserving coatings is dry-film protection, which protects coatings from microbes in application areas such as in bathrooms, kitchens, and on exterior surfaces. Lastly, plant hygiene is critical for coatings preservation. If a tank or a pipe becomes contaminated, it can contaminate the final product. Each of these three aspects requires a different approach for preservation. From the consumer’s perspective, the biocides that are present in the final product are the most important: both for in-can and dry-film preservation. Ideally, antimicrobial materials would maximize efficacy, while minimizing toxicity and environmental persistence. Active antimicrobial ingredients need to be stable within the shelf life of the product to maintain product quality, but also biodegradable when exposed to the environment to deliver eco-friendly products. Furthermore, they need to be effective against microbes but non-toxic to other life forms. Balancing these needs is difficult to achieve in reality.
Regulations Necessitate New Preservation Methods
Due to their potential for human and environmental toxicity, antimicrobial active ingredients are heavily regulated by government agencies. The preservation options currently available to the paint and coatings industry for both in-can and dry-film preservation are narrow due to increasing regulatory restrictions. Currently, the main biocidal actives used by paint manufacturers belong to the isothiazolinone family. However, this class of chemicals is under increasing pressure globally as a result of concerns for dermal sensitization. The changing regulatory, eco-labeling landscape, as well as consumer pressure globally, may require new ways of preserving most coatings raw materials as well as paints in the wet and dry states.
The changing regulatory landscape also brings new opportunities for raw materials suppliers to provide innovative solutions for coatings preservation. Researchers have been working on developing binders and additives that can help coatings formulators greatly reduce or eliminate the need for biocides for in-can preservation. Although there are high pH (> 10–11) silicate paints on the market that claim to be biocide-free, we are focusing on providing solutions that enable coatings manufacturers to formulate their final coatings under a traditional pH range (pH 7–10).
Commercially Available Solutions Are Limited
The majority of commercially available biocide-free coatings are high-pH formulations, where the pH > 10–11 conditions are antagonistic to microbial growth.1 The high-pH paints are largely based on inorganic silicate binders. Although inorganic, silicate-based paints are biocide-free and durable for exterior application, these coatings are limited to certain substrates, including masonry, mineral plasters, and concrete coatings. Moreover, the corrosive nature of the pH > 10–11 paints has the potential to be harmful to consumers and may require protective equipment, such as goggles, for application. In addition to the high-pH inorganic paints, another route to biocide-free paints is through the use of super-hydrophobic ingredients to reduce microbial growth in architectural and marine anti-fouling coatings. Super-hydrophobic coatings are more often used for dry-film preservation. Based on fluorine or silicone technology, super-hydrophobic coatings resist microbial growth by slowing or preventing the surface adsorption of microbes. This technology works better in marine anti-fouling coatings where the ships’ motion through water helps clean the surface. Surfaces that are cleaned less often, such as fences or fixed exterior structures, may have larger challenges in using super-hydrophobic coatings to act as the sole dry-film preservative. A further challenge to using fluorine chemistry is that it is generally not readily biodegradable, which could potentially cause bio-accumulation and persistence concerns.
There are commercially available technologies that offer solutions that are free of 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT) or 2-methyl-4-isothiazolin-3-one (MIT) to paint manufacturers through biocide degradation, which are limited in scope. The so-called CMIT-killer does not remove MIT from the formulation, which is particularly important because CMIT is usually sold in a 3:1 ratio with MIT in current commercial biocide formulations. An alternative way to remove MIT from coatings and coatings raw materials is to develop an MIT-killer in addition to a CMIT killer, or, if available, use a CMIT-only product that has just recently become available. Once CMIT and/or MIT is degraded, however, the paint manufacturer is left with inadequately preserved materials, which is the second drawback to the biocide-killer technology. Biocide killers do not replace the effective biocide MIT or CMIT/MIT with a good alternative preservation option. The crux of the problem is that there are limited commercially available biocides approved for use in coatings that are inexpensive, compatible, environmentally friendly, non-toxic, and effective in all raw materials and paints, and those that do exist have uncertain regulatory futures.
Examples of commercially available, non-sensitizing biocidal chemistries registered for coatings use include cationic nitrogen-based, silver-based, or zinc-based biocides. Quaternary/cationic nitrogen amines have been used in commercial paints as biocides. As the majority of waterborne coatings are anionically stabilized, it is challenging to incorporate the cationic biocide into a formulation. Silver ion is a well-known antimicrobial agent used in the textile and coatings industries to inhibit microbial growth. It is more commonly used for dry-film preservation, which only requires the actives to be present at the surface. For in-can preservation, the concentration of silver ion needs to be high enough to inhibit microbial growth in the wet state. Due to the high cost of silver, using silver ion technology for in-can preservation of coatings has been limited. Zinc complexes are other potential preservation candidates. For example, zinc oxide has been used as a dry-film preservative in exterior coatings to reduce fungi and algae growth. However, zinc oxide and other zinc complexes are not without controversy, given the recent opinion of the risk assessment committee of the European Chemicals Agency (ECHA) to classify zinc pyrithione as a repro-developmental toxicant.
Emerging Technologies Offer Potential
In addition to the above-discussed solutions that are approved for coatings preservation, there are also a few emerging technologies that could be the next-generation biocides. The first technology direction is biological-based solutions. Several start-up companies have looked into sensitizer-free preservatives for consumer products. Examples include kimchi fermentation peptides or an amino acid-based antimicrobial system.2-3 These actives are not yet approved for coatings use. In addition, it might be too expensive for coatings use and the antimicrobial efficacy in various coating raw materials and formulations still needs to be validated.
A second future direction is antimicrobial polymers, which incorporate functional ingredients into a polymer structure. This alternative avoids the use of lower molecular weight biocides, which can reduce the sensitization potential and improve the long-term effectiveness. The antimicrobial properties can be achieved either through the use of a functional monomer in the polymerization or post-functionalization of the polymer. A variety of functional groups could be incorporated into the polymer, including cationic nitrogen, halogen, phospho/sulfo derivatives, phenol and benzoic derivatives, organometallic groups, etc.4
The last direction for biocide-free coatings is through packaging. For example, anti-septic packaging is commonly used in the food industry. To achieve biocide-free materials via anti-septic packaging is challenging for the coatings industry, as it requires significant investment in plant hygiene and packaging materials. In addition, it implies a new model of coatings supply and consumption. Once the paint can is opened, it will be susceptible to microbial contamination. Thus, consumers will not be able to preserve the paint for future use, and wastage will likely be increased significantly.
Development of Robust Raw Materials for Standard pH Coatings
Experimental
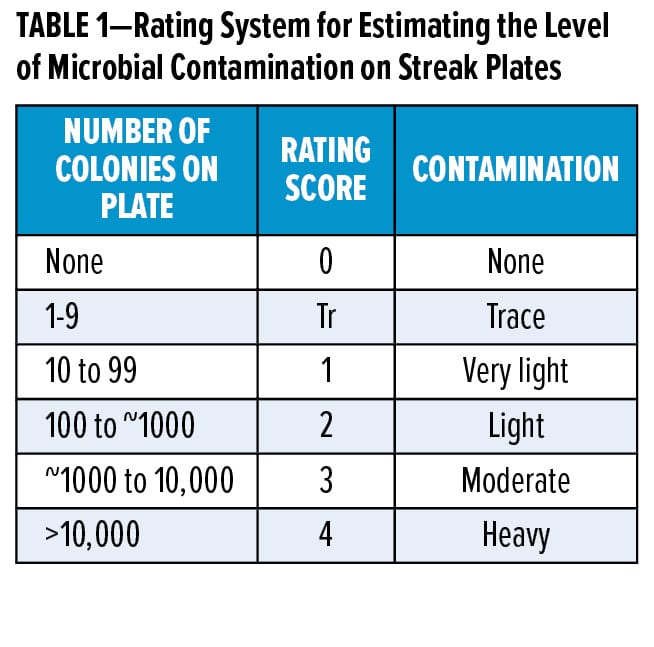
Raw materials and model formulations were tested for microbial growth susceptibility using a series of challenge tests. Samples were inoculated two times at seven-day intervals with 106–107 colony-forming units per milliliter of sample (CFU/mL) of a standard pool of bacteria, yeasts, and molds that are common contaminants in coatings. Test samples were monitored for microbial contamination by agar plating using a standard streak plate method. Samples were plated one and seven days after each microbial challenge onto trypticase soy agar (TSA) and potato dextrose agar (PDA) plates. All agar plates were checked daily up to seven days after plating to determine the number of microorganisms surviving in the test samples. The degree of microbial contamination was established by counting the colonies, where the rating score was determined from the number of microbial colonies observed on the agar plates (Table 1). Reported results come from day seven readings.
For the experimental binder samples, Challenge 1 included a standard pool of bacteria, yeasts, and molds. Challenge 2 had additional Gram-positive bacteria field isolates; and Challenge 3, where performed, consisted of a field isolate yeast strain from a western European coatings facility and further gram-positive bacteria. For all other challenge tests, samples that had no to very light microbial contamination after the first two inoculations with the standard pool of microbes (Challenge 1 and Challenge 2) were then further challenged with the same microbial pool in Challenge 3. The more complex microbial pool used in Challenge 3 was intended to test real-world microbes found in manufacturing plants and to increase the difficulty of the challenge.
Binders More Robust Against Microbial Spoilage
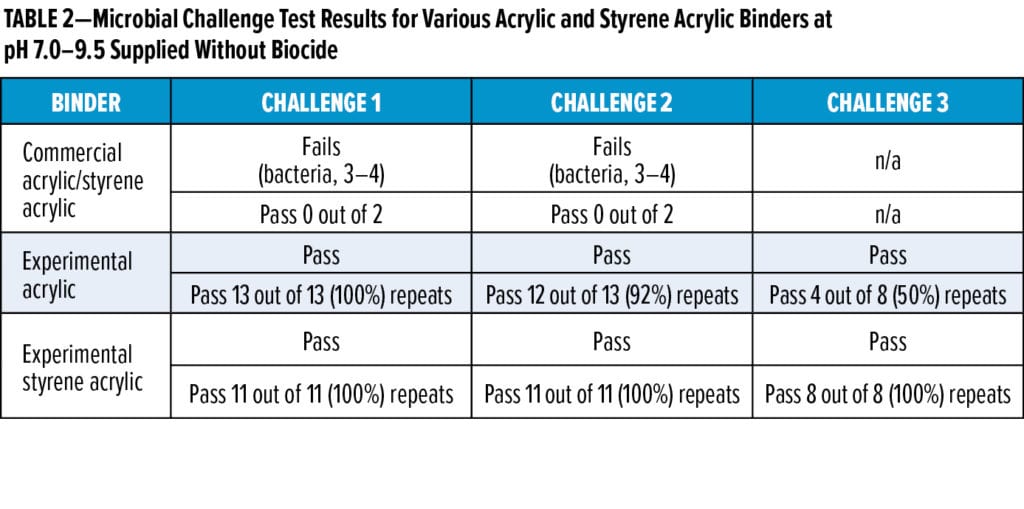
Typical commercial waterborne acrylic and styrene acrylic coatings binders are susceptible to spoilage caused by microbial growth when no biocide is added (Table 2). Today, there are few commercial alternatives to biocides such as CMIT, MIT, and BIT (1,2-benzisothiazolinone) that are effective at maintaining the binder’s quality over the product’s life cycle. To enable biocide formulation flexibility and biocide-free coatings, we developed experimental REACH-compliant acrylic and styrene acrylic binders that are inherently less susceptible to spoilage without biocide addition. The experimental binders greatly reduce the risk of microbial spoilage compared with a typical commercial acrylic or styrene acrylic binder. For example, when biocide-free commercial acrylic or styrene acrylic binders were inoculated with a standard pool of microbes, they failed all challenge tests with growth ratings of three to four for bacteria and fungi (Table 2), while the experimental acrylic and styrene acrylic binders passed multiple repeated challenge tests, including Challenge 3, in 50% and 100% of the tests, respectively (Table 2). Further experimental derivatives were also more robust against microbial growth. These findings indicate that specialty binders that are less susceptible to microbial spoilage can be designed for the coatings space.
Paint Formulated with Experimental Binders Passes Challenge Tests
To enable biocide-free coatings, the binder, once formulated into a paint, also needs to have lower susceptibility to microbial growth. As a result, microbial challenge tests were also performed on 15% to 30% pigment volume concentration (PVC) and 35% to 40% volume solids paints composed of titanium dioxide, surfactant, experimental binder, and water. When tested by itself, the grind failed microbial challenge tests with a 3 rating, while the paint formulated with the experimental binders passed both standard pool Challenge Tests 1 and 2. Although this study lacks the complexity of a fully formulated paint, it indicates that the experimental binders are promising candidates to enable coatings manufacturers to reduce or, in certain areas, eliminate in-can preservatives in their formulations.
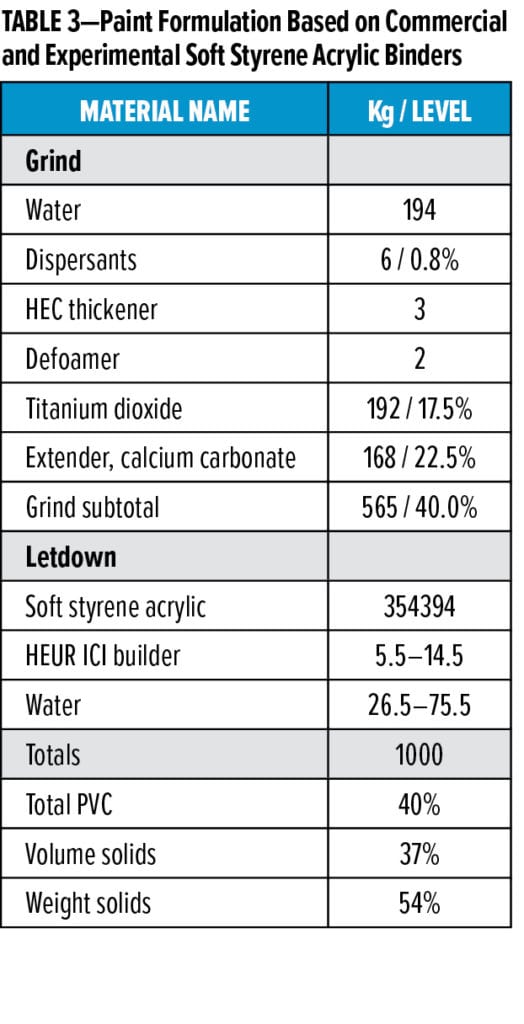
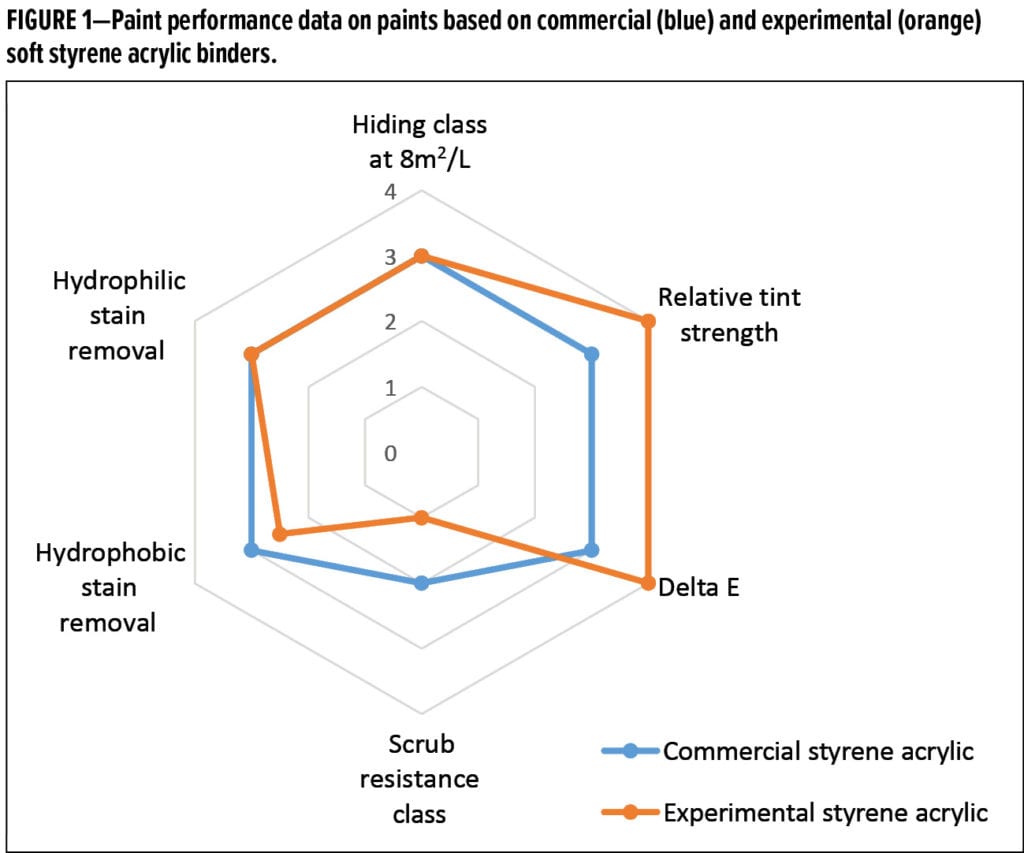
In addition to being less susceptible to microbial growth, the experimental acrylic and styrene acrylic binders must deliver excellent dry-film properties, as would be expected in a typical commercial binder. To demonstrate the experimental binder’s utility, we compared the performance of commercial and experimental soft styrene acrylic binders in a 40 % PVC, 37% volume solids paint formulation thickened to 110–115 KU Stormer viscosity, 1.0–1.3 P cone and plate ICI viscosity, and 6000–7000 cP Brookfield viscosity measured with spindle 4 at 60 rpm (Table 3). Overall, the experimental binder functioned well in a coatings formulation as Figure 1 shows. Lower ratings indicate better performance. The hiding class was measured using ISO6704-3 method using contrast ratio, and scrub class was determined using the ISO11998 method by weight loss in µm at 50°C and room temperature.
HEUR Rheology Modifiers that Are Less Prone to Microbial Spoilage
Hydrophobically modified ethylene oxide urethane (HEUR) thickener products are typically preserved with biocides to maintain product quality and safety because bacteria, yeast, and mold can thrive in traditional low-VOC HEUR rheology modifiers (Type 1), which are in the range of 5 < pH < 8. To ensure product quality without the use of biocides, the rheology modifier solutions themselves need to become inhospitable to bacteria and fungi (which includes yeast and mold).
The two main approaches to accomplish this goal are either through the thickener formulation or the HEUR polymer itself. The thickener formulation can be optimized to reduce the potential for microbial growth. For example, alcohols and glycols are well-known disinfectants. These are often used in HEUR solutions to lower the viscosity, but they are also helpful in preventing microbial growth. Unfortunately, this solution can contribute unwanted VOCs to the coating. Another common formulating lever to reduce microbial growth potential is a pH above or below which most microbes can grow.1 Finally, the HEUR polymer itself can be designed to become less susceptible to microbes.
To reduce the microbial susceptibility of traditional Type 1 HEURs (shown in Figures 2a and 2b), we first attempted to make the HEUR formulation less hospitable to microbial growth by lowering the pH of the solution to 2.1–4.0 with a variety of acids. Table 4 shows that while lowering the pH of Type 1 HEURs reduces the microbial growth rating of the polymer from a 3–4 rating to a 2 rating (10–100 times less growth), it is insufficient to protect the HEURs from bacteria, yeast, and mold. A pass in Table 4 indicates no growth on day seven. We further reduced microbial susceptibility by altering the HEUR polymer itself to create specialty experimental HEUR Type 2 (Figure 2c). This modification of the HEUR polymer reduces the spoilage potential beyond that of the traditional Type 1 HEURs at the same low pH, where Type 2 HEURs were only susceptible to mold growth. Finally, further optimization of the experimental Type 2 HEUR (Type 3) can yield solutions that pass our challenge tests, including mold, to at least three test cycles (Table 4, Figures 2d and 2e).
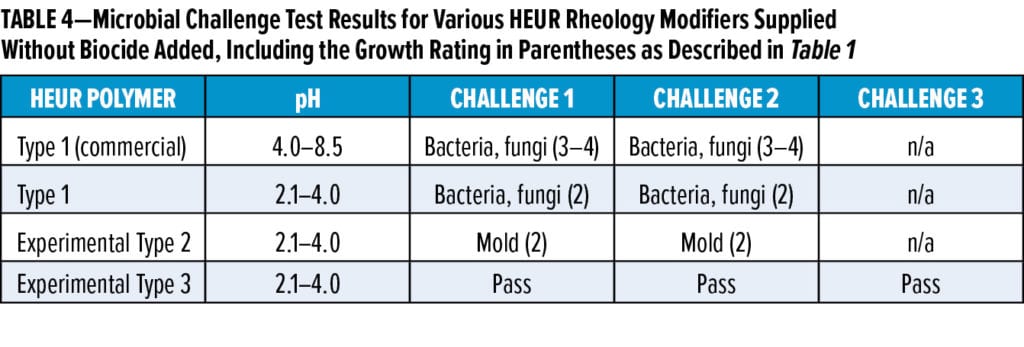
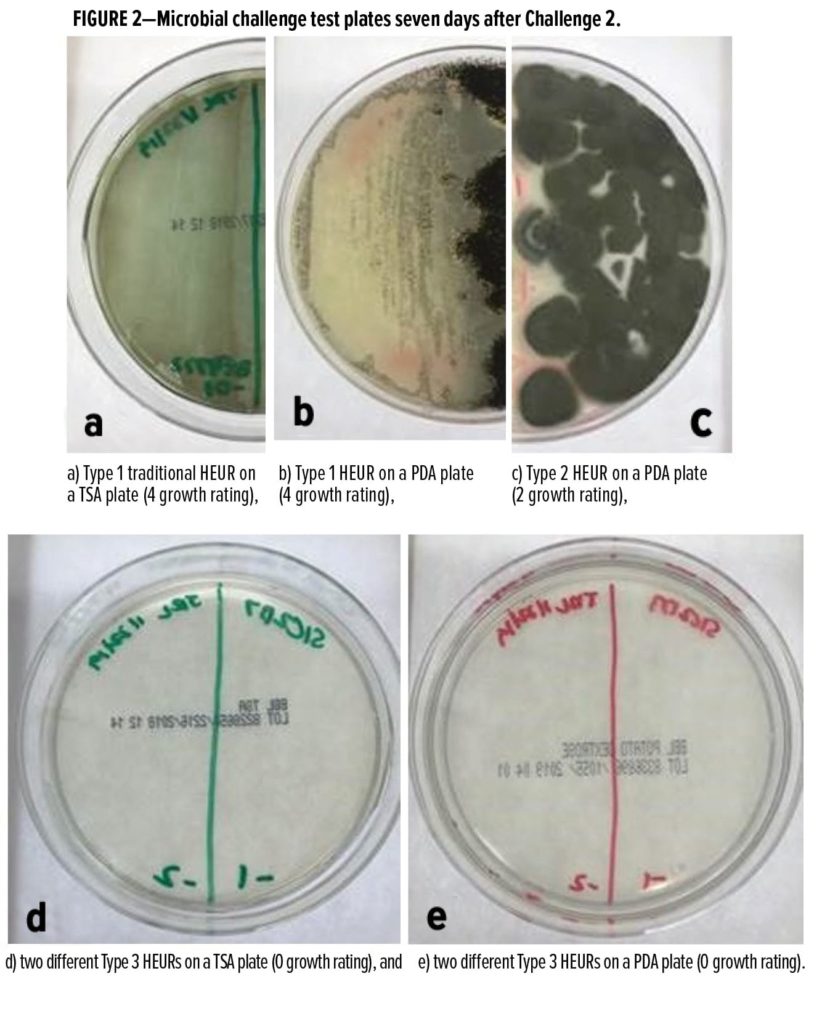
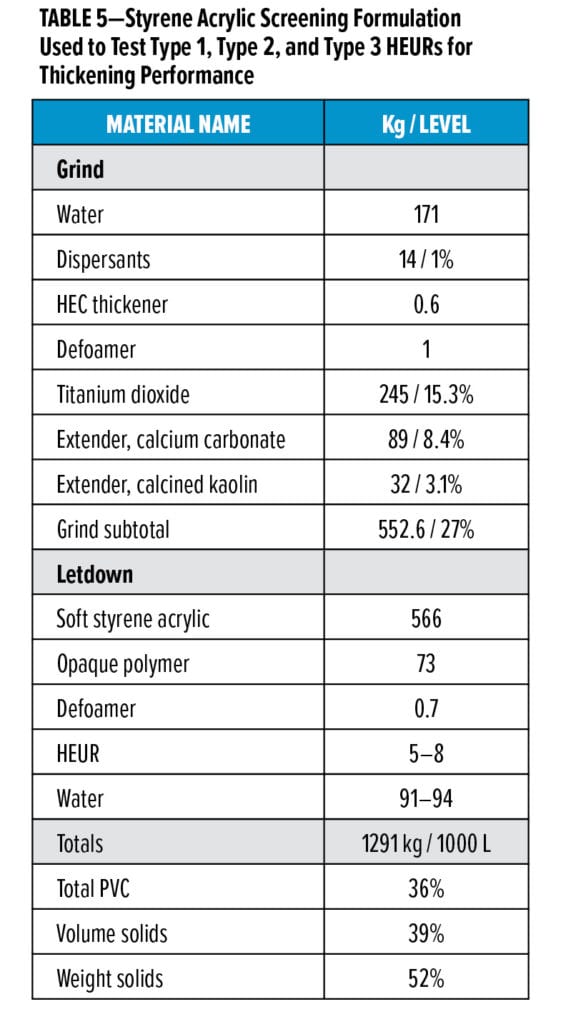
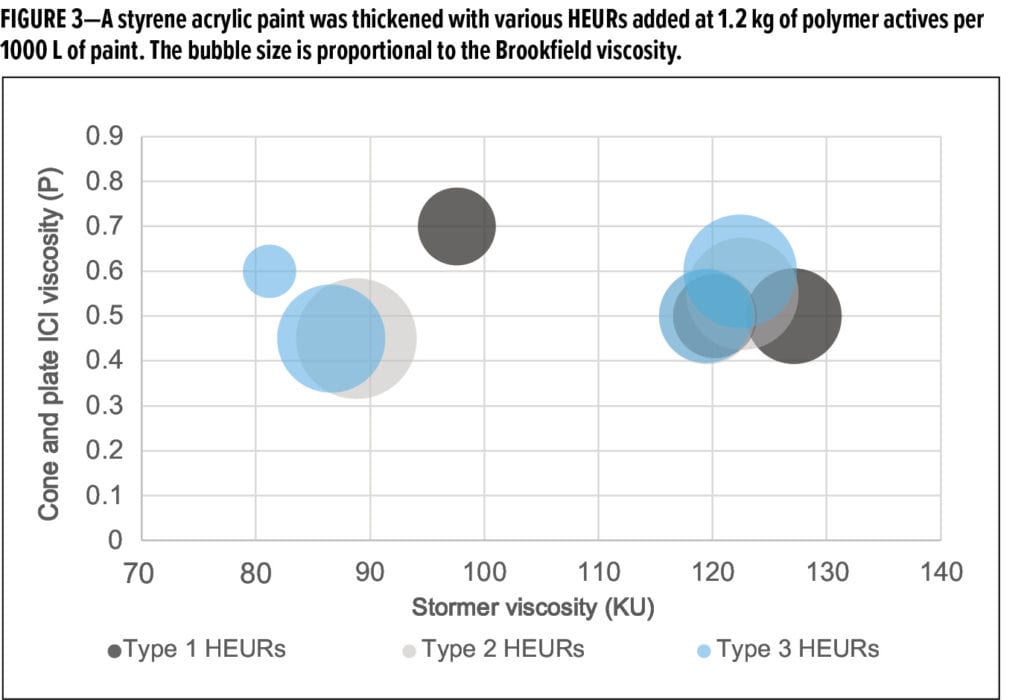
Despite optimization of the polymer and solution to reduce susceptibility to microbial spoilage, the experimental Type 2 and 3 thickeners can still provide the high thickening efficiency and desirable rheology performance of a traditional, but growth-prone, Type 1 HEUR. Table 5 presents the styrene acrylic screening formulation used to test the thickening performance. Figure 3 illustrates the stormer viscosity of a 36% PVC, 39% volume solids styrene acrylic paint thickened with various Type 1 (black), 2 (gray), and 3 (blue) HEURs added at 1.2 kg of polymer actives per 1000 L of paint. The bubble size is proportional to the Brookfield viscosity.
Path to Reducing Spoilage Without Biocides
Experimental acrylic and styrene acrylic binders and experimental specialty HEUR rheology modifiers have lower potential for microbial spoilage than traditional products. We have developed several experimental binders that pass microbial challenge tests and have formulated these binders into simple paints that also pass challenge testing. By optimizing the HEUR polymer and formulation, the HEURs were able to pass at least three challenge tests. Together, these results provide encouragement that there is
a path forward to create sustainable and robust raw materials that can reduce spoilage without the addition
of biocides.
References
- Rosso, L., Lobry, J.R., Bajard, S., and Flandrois, J.P., “Convenient Model to Describe the Combined Effects of Temperature and pH on Microbial Growth,” Applied and Environmental Microbiology, 61, 610–616 (1995).
- Hodges, T.W., et al. “Proteins and Peptides as Replacement for Traditional Organic Biocides: Part I,” CoatingsTech, 15 (4), 44–50 (2018).
- Crovetto, A., In-Cosmetics Global Presentation. Transforming the Face of Preservation: Peptide Technology in the Personal Care Industry (2018). Available at: https://www.in-cosmetics.com/RXUK/RXUK_InCosmetics/
2014-website/Documents/Active%20Concepts%20Presentation.pdf?v=635340184796784783. - Muñoz-Bonilla, A., Fernández-García, M., “Polymeric
Materials with Antimicrobial Activity,” Progress in Polymer Science, 37, 281–339 (2012).
