By Douglas M. Lamb, Coatings Consultant
The Birth of Automotive Coatings
“The customer can get the Model T painted in any color he wants, so long as it’s black!”—Henry Ford, 1908.
The oft-quoted statement above of Henry Ford is humorous today, but Ford was serious when he said it. In 1908, Ford thought that black car paint was the only practical automotive paint for the Model T, as it provided him with a coating that was both durable and cheap in cost. Of course, the black car paint that Ford put on his Model T actually was not “automotive” paint at all, but just the existing paint technology available at the beginning of the 20th century: a paint based on natural linseed oil resin as the binder. Oil resins cure through oxidative crosslinking, which means the paint takes a long time to dry. Ford’s black paint was applied by hand brushing to the Model T in multiple coats, a process that, in the end, took about a week to complete. This caused a terrible production bottleneck for Ford’s innovative mass production process, even though the black paint dried faster than all other available colors. Model Ts undergoing the painting process at the end of the assembly line jammed warehouse floors of the automotive plant.
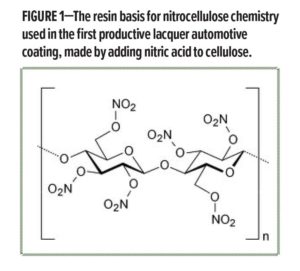
This process bottleneck was the motivation for the first paint specifically developed as an automotive coating: DuPont Company’s “Duco” paint. This new coating technology made a step change in productivity by reducing the painting and drying time from many days to a few hours. The DuPont chemists who had used nitrocellulose chemistry (Figure 1) to develop explosives and motion picture film found that if they modified the molar ratio of the NO2 groups in the cellulose backbone, they obtained a low viscosity lacquer resin at about 15% resin solids that could be spray applied as a coating. Being a lacquer, this coating dried (merely through solvent evaporation) in about two hours. Some formulation development work by the paint chemists found that this new synthetic lacquer resin provided an excellent basis for a paint that had improved appearance, toughness, and durability versus natural oil resin paints, and also could be easily pigmented with a wide variety of color pigments, besides just black! After a couple of years of testing, in 1924 General Motors introduced the use of Duco finishes on almost their entire automotive line.
This was the first example of how industry needs have driven the advance of automotive coatings technology. In this case, the need for improved productivity in the automotive plant drove the invention and development of a new coating chemistry. Since that first new technology advance in the 1920s, innovations in automotive coatings technology have continued unabated. In fact, many of the new technologies and chemistries in coatings science have come from advances pioneered in the automotive coatings field. This article is intended to provide a brief historical survey of the evolution of automotive coatings technology.
Early Automotive Coatings Chemistries: From Alkyds to Acrylic Lacquers
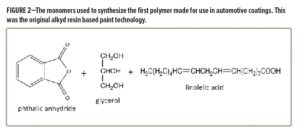
Nitrocellulose paint was highly productive, but the final coating required polishing to achieve high gloss. Paint chemists in the 1930s wondered if somehow they could find a binder system for paint that provided both productivity and the inherently better appearance of a natural oil resin. What resulted from this work was the development of the first alkyd paint system. This was the first “polymer” made for coatings, as it was synthesized using three monomers: phthalic anhydride, glycerol, and linoleic acid (Figure 2). In this way, the chemistry combined both synthetic monomers and natural products, providing a coating resin system that gave intermediate performance between synthetic lacquers and natural oils. Given that this technology provided outstanding film properties, this new alkyd paint was first commercialized as an automotive primer. Important to coatings science, note that in this case a resin technology was chosen for a specific coating layer of a total coating system, a fundamental concept used in coatings today. Additionally, alkyd chemistry continues as a mainstay of current coating technology.
It was not until the 1950s that the next major automotive coatings advance occurred: the use of thermoplastic acrylic lacquers. By this time in American society, the automobile was no longer just a means of transportation; cars had now become a personal showpiece that owners wanted to show off to their friends. That meant that the coatings had to look better and accentuate the new curved styling body designs of the times. Rohm and Haas Co. had developed a new synthetic polymer as a glass replacement based on poly methyl methacrylate, and the coatings industry investigated whether that technology could be used in coatings. This chemistry, of course, is based on the controlled polymerization of various acrylic monomers, to obtain a polymer resin of desired molecular weight and glass transition temperature. This would be the first all man-made resin technology to be used in automotive coatings.
It turned out that thermoplastic acrylic resin technology dominated the automotive topcoat market in automotive coatings for about two decades, from the 1950s through the 1970s. The reason for this was the excellent topcoat appearance that could be obtained with these finishes. The acrylic resin binder was high in viscosity, given its high molecular weight (80–100k) and high Tg (approximately 70°C). Thus, coatings based on this technology needed to be sprayed at relatively low solids of about 20%. In the automotive plant, this meant multiple coats of the topcoat were applied to reach the desired film build of about 2 mils. By today’s standards, this sounds like a disadvantage, but at the time, this acrylic lacquer technology had one critical advantage over previous automotive paints: it provided an excellent binder system for the newest pigment colorant technology—metallic pigments.
Metallic effect pigments provide brilliant, shiny car colors that enhance the perception of curvature of the car body. These pigments took automotive color styling to a new level. However, to achieve the maximum visual effect of the flat, plate-like metallic pigments, the pigments must align parallel to the painted surface. The rheological profile of an acrylic lacquer paint is perfect to obtain this effect: a low initial viscosity (given the low solids) to allow the metallic flakes to lay flat, and then a fast rise in viscosity (given the high molecular weight and Tg ) to keep the flakes in place. This coating technology had such an advantage for color styling that by the 1960s General Motors painted virtually every car with acrylic lacquer topcoats.
Protecting the Automotive Body
Topcoat technology was steadily improving for automotive coatings systems, but cars still had a major issue—rusting of the automotive body. A major coatings advance in the 1970s resolved this issue: electrodeposition primers, commonly known as “e-coat.” The first automotive electrocoat was an anodic product developed by Dr. George Brewer at Ford around 1957. However, there were drawbacks in the technology and PPG Industries introduced the first cathodic e-coat system for automotive bodies in 1973. Because these coatings essentially stop the automotive body from rusting, this new primer technology one was of the biggest breakthroughs in automotive coatings technology.
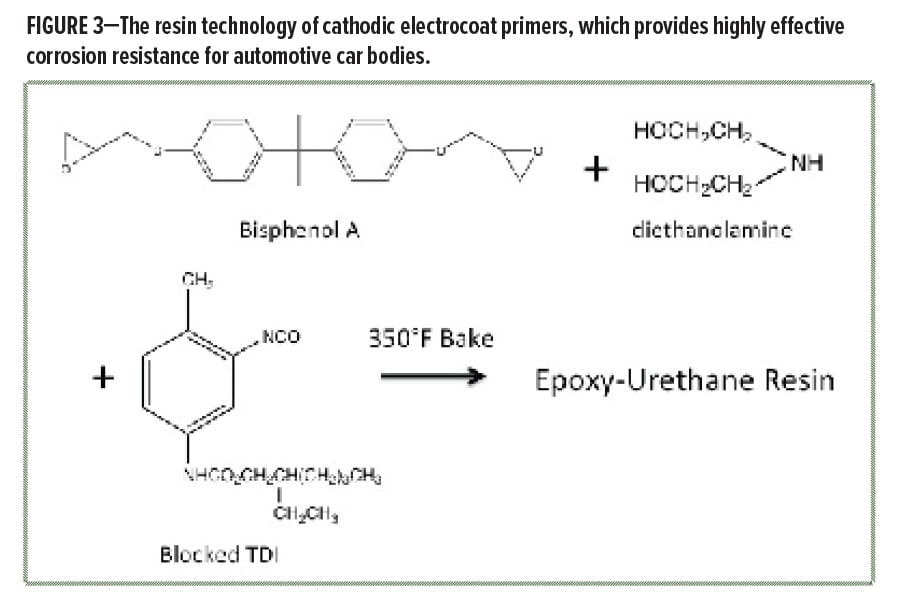
Modern electrocoat automotive primers are applied by totally submerging the assembled car body in a large tank that contains the waterborne e-coat, and the coating is applied through cathodic eletrodeposition. This assures nearly 100% coverage of all metal surfaces by the primer. The coating chemistry is waterborne enamel based on epoxy, an aminoalcohol adduct, and blocked isocyanate, which all crosslink on baking to form an epoxy-urethane resin system (Figure 3). This resin technology, combined with the excellent coverage provided by electrodeposition, delivers one of the most effective coatings for corrosion protection known. Virtually all cars use e-coat technology as the foundation of their coating system today.
Although e-coat provides excellent corrosion protection, it does have two weaknesses for an automotive coating system: inadequate appearance and poor photostability. To remedy these issues, new enamel automotive primers were developed in the 1980s. These primer-surfacers were designed to be applied to the cured e-coat to give a smoother surface for improved topcoat appearance, while also providing opacity to protect e-coat primers from UV degradation. Primer-surfacers often provided improved impact resistance to reduce stone chipping of the coating as well. The combination of electrocoat plus primer-surfacer provided a total automotive primer system with excellent corrosion protection and an outstanding surface for topcoating. That set in motion the next major breakthrough in automotive coatings technology: basecoat/clearcoat topcoats.
Basecoat/Clearcoat Automotive Topcoats
As previously discussed, thermoplastic acrylic lacquer automotive coatings, given their excellent appearance, were the major automotive topcoat used in the 1950-70s. However, these lacquer topcoats did have one significant drawback: they had weak exterior durability. After about one to two years’ exposure, the coatings would begin to degrade, and aggressive waxing was needed to “bring back the shine” of these systems. By the 1980s, the automotive manufacturers were requesting better durability for automotive topcoats, as consumers were now expecting their cars to last at least five years, and they wanted the car to continue to look like it did when they first saw it in the showroom. At the same time, the Environmental Protection Agency began to promulgate new volatile organic compound (VOC) regulations that limited the amount of solvent that an automotive facility could emit into the atmosphere. The high VOC content and weak durability of acrylic lacquer coatings were no longer acceptable in the automotive marketplace.
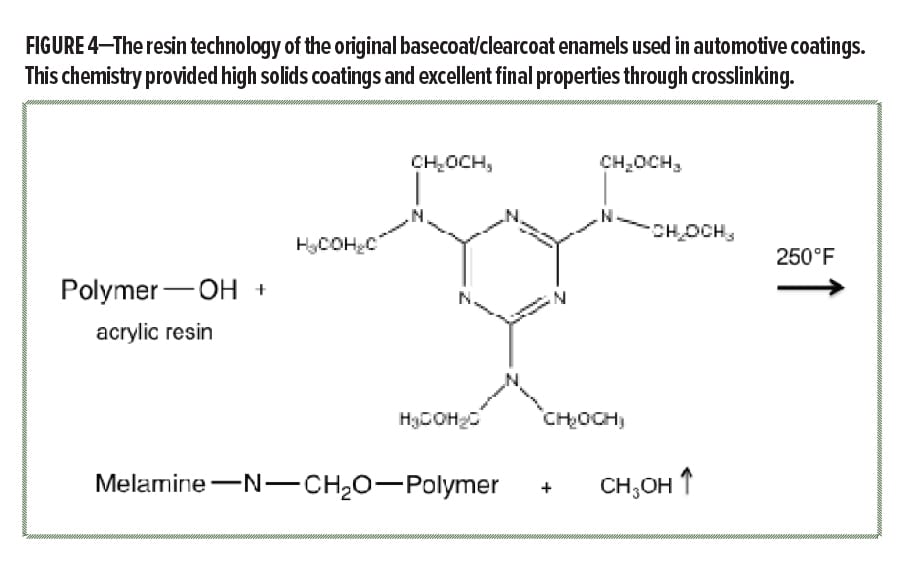
So how did the automotive coatings formulators achieve higher solids, better durability, while even improving the appearance of the coating? The answer is the next step change advance that occurred in automotive coatings: basecoat plus clearcoat enamel topcoat technology. Now, instead of a single layer topcoat, formulators designed a two-layer system consisting of a basecoat that contained the pigments to provide the beautiful color effects, followed by a clear polymer coating layer that protected the basecoat. Both the basecoat and clearcoat were enamels, which were based on hydroxyl-functional acrylic resins crosslinked using melamine chemistry (Figure 4). This new concept of basecoat/clearcoat enamel topcoats had many advantages: (1) reduced solvent content was achieved given the low molecular weight of the hydroxyl-functional acrylic resins (approximately 10k) and low viscosity melamine crosslinker, (2) the coating cured to a high crosslink density on baking to provide better properties, (3) the unique basecoat layer allowed the use of even more effect pigments by the color stylists, and (4) the clearcoat layer was formulated to provide both better appearance and the ultimate in protection for the coating system below. These basecoat/clearcoat systems were able to achieve a previously unattainable balance of properties for the automotive coating system, providing stunning visual appearance and long-term durability.
Waterborne Basecoats, New Crosslinking Chemistries, and New Application Processes
In the 1990s, another major development occurred in the formulation of automotive coatings: the use of waterborne basecoats. The chemistries of these basecoats can vary from water-reducible acrylics and polyesters, to acrylic latexes, to polyurethane dispersions, but the common factor is the use of water as one of the main volatile components. Typically, the motive to use waterborne technology is to obtain lower VOCs and reduce the environmental footprint of the coating process, but that is not the only benefit of using waterborne basecoats in automotive. It turns out that waterborne automotive basecoats, given their lower formulated solids and unique rheology profiles, can often provide improved appearance and metallic effects. Thus, in some ways, the move to waterborne basecoats in automotive can be thought of as a return to the low-solids acrylic lacquer topcoats of the 1950s.
Automotive coatings using many new crosslinking chemistries have also been developed over the last two decades. Clearcoats have been the focus for these new chemistries, so in addition to the original acrylic resin/melamine systems, there are now acrylic/silane/melamine, acid/epoxy, carbamate/melamine, and acrylic/isocyanante systems. Important new properties for clearcoats can be achieved with these new crosslinking chemistries, such as improved appearance and durability, better acid etch resistance, and scratch and mar resistance.
The final major step change in automotive coatings technology occurred in the 2000s, and this advance focused on process efficiency. In the typical automotive assembly plant, the painting operation can take up to half the space of the entire facility, account for approximately 40% of the capital cost of an assembly plant, use 80% of the energy, and produce the vast majority of CO2 and VOC emissions! OEM manufacturers have asked the paint suppliers to find a way to reduce this footprint and the cost of applying the coating system. This has required paint formulators to develop coatings that can be applied more efficiently, in fewer steps, and with a lower energy requirement.
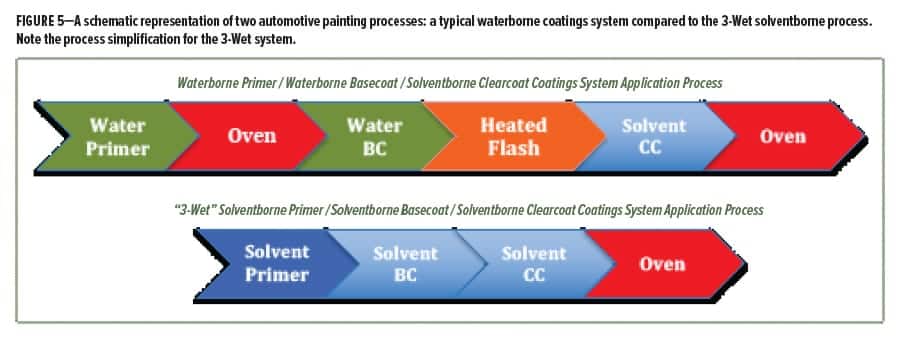
Many new processes are now in place at the automotive plants that meet these criteria. As an example, Figure 5 compares a diagram of a typical automotive primer/topcoat paint system process versus the newest coatings process that Ford has implemented in many of its assembly plants. The typical process for a waterborne primer, waterborne basecoat, and solventborne clearcoat system includes two oven bakes and a heated flash, all of which take time and energy. Compare that to the new “3-Wet” process at Ford, where solventborne primer, solventborne basecoat, and solventborne clearcoat are applied one after the other, and a single bake is performed after the application of all three layers of the coatings system. This 3-Wet application method reduces the footprint of the coatings line, shortens the overall time of the painting process, and saves energy costs. Interestingly, most of the energy savings arise from the elimination of the primer booth not the primer oven. The movement of up to several hundred thousand cubic feet per minute of conditioned (temperature and humidity) air through a paint booth consumes much more energy than the natural gas used to heat an oven. Other “compact” processes have been introduced by other automotive manufacturers and use names like B1/B2 or 3-coat 1-bake. All essentially remove the stand-alone primer booth and oven, which results in significant savings without compromising quality. Clearly, these changes in application process require reformulation of the primer/topcoat coating system to tolerate the wet-on-wet processes.
It is interesting to note that I began this history of automotive coatings technology with a discussion of the development of a new coating to improve productivity of Henry Ford’s black paint, and now—a century later—the latest advances in automotive coatings technology have returned to focusing on that goal.
CoatingsTech | February 2017 | Vol. 14, No. 2