By , Ph.D., The ChemQuest Group
Anyone who has had the pleasure of scraping ice off a car windshield, looking nervously at beautiful but deadly ice spears hanging from winter rooftops, or waiting on a slow and interminably long aircraft deicing line at an airport has dreamed of having some sort of coating that would eliminate inconvenient ice and snow accumulation. The desire to triumph over ice and snow accumulation has, in fact, been the focus of significant technical work that has been going on since the 1940s in an attempt to make all kinds of surfaces ice and snowphobic.
Icephobicity is defined as the ability of a solid surface to repel ice or prevent ice-film formation due to topographical structures at the ice-surface interface. Ice-repelling characteristics are attributed to the surface structure (sometimes called surface roughness) and/or low surface energy leading to poor ice adhesion allowing for easy removal.
Icephobic coatings are formulated to ensure that ice doesn’t accumulate on or strongly attach to a surface. In fact, fully functional icephobic coatings operate on several different levels. They can have the ability to repel water droplets, delay ice nucleation, and/or reduce ice adhesion.
Icephobic performance is evaluated using two comparative test methods. The ice Centrifuge Adhesion Test (CAT) measures the adhesive failure between the ice and the surface–in other words, the force required to separate the ice buildup from the coating is measured. In this test, a bare aluminum reference and a coated aluminum sample are simultaneously iced by supercooled precipitation. The ice-adhesion shear stress is calculated from the ice-detachment rotation speed. The results are reported as the Adhesion Reduction Factor (ARF), which is the ratio of ice-adhesion stress on the bare aluminum and the ice adhesion stress on the coated sample.
The second test method is the Static Ice Accumulation Test (SAT), which measures the amount of ice that accumulates on uncoated reference and coated substrates that are placed at angles of 45° and 80°. The results, reported as the Accumulation Reduction Factor (ACCRF), are the ratio of the ice mass on the bare reference sample over that of the coated sample.
Fully functional icephobic coatings operate on several different levels. They can have the ability to repel water droplets, delay ice nucleation, and/or reduce ice adhesion.
Over time, a wide range of ARF values, from 0.5 to 1000, have been reported, that correlate to interfacial stresses of 800 kPa and 0.5 kPa respectively.1
Several properties influence icephobicity. Hydrophobicity in particular prevents water droplets from attaching to and penetrating through the surface. Hydrophobicity is a function of water-contact angle. As shown in Figure 1, three levels of hydrophobicity are defined for contact angles >90°.
FIGURE 1. Hydrophobicity as defined by liquid contact angle.2

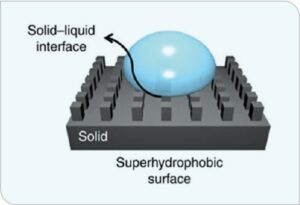
Superhydrophic surfaces (Figure 2) have strong water-repulsion properties that also decrease ice-nucleation temperatures or increase time to ice nucleation, leading to lower ice-adhesion strength.
FIGURE 2. Schematic of a superhydrophobic surface.3
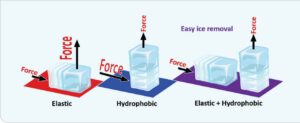
The reduction of ice-adhesion strength, known as interfacial cavitation, is the critical factor in determining the force in removing an ice crystal from the surface. This force is not trivial, as ice adhesion can be as high as 1600 kPa on an untreated surface. Conversely, icephobic surfaces exhibit ice-adhesion forces of 100 kPa or less. Figure 3 illustrates the forces necessary for ice detachment. Detachment mode, shear-mode deformability, and tensile-mode hydrophobicity all impact the adhesion strength. Oil infusion further reduces ice adhesion due to interfacial slippage.
FIGURE 3. Ice adhesion depends upon the detachment mode, deformability in shear mode, and hydrophobicity in tensile mode.4
The concept behind interfacial cavitation is that a soft surface (polymeric elastomer, for example) attaches to a hard surface (ice). The elastomer deforms while the ice remains a hard, inflexible mass causing a stress concentration at the interface, making the ice break free. This was developed accidentally when it was discovered that different adhesion strengths can be tailored for the same polymer. Adhesion strengths as low as 0.1 kPa can be achieved with some of these coatings–although at that level they may be too fragile.
Continue reading in the January-February 2024 digital issue of CoatingsTech.
