By Ram Lalgudi, Aries Science & Technology
The need for green and environmentally safe coatings creates an opportunity for biobased raw materials. Vegetable oil polyols (VOPs) are the major source of biobased raw materials for making polyurethanes1.
Despite their wide commercial availability, they have not been utilized for making water-based polyurethane (PU) coatings, because, VOPs have no performance-enhancing functional group in the molecular architecture2. Therefore, they are unable to meet the stringent corrosion-, UV-, and solvent-resistance performance requirements.
In this short communication, we present a new class of biobased polyol with a crosslinking functional group that is free from toxic chemicals and has superior performance. This is achieved by reacting a keto acid with a glycidyl functional fluids derived from biobased sources. We also present the utilization of the polyol in making water-based polyurethane dispersion (PUD).
SYNTHESIS OF THE POLYOL
In a typical example as shown in Scheme 1, levulinic acid is reacted with glycidol to produce 2,3-dihydroxypropyl levulinate (Levupol-G ).
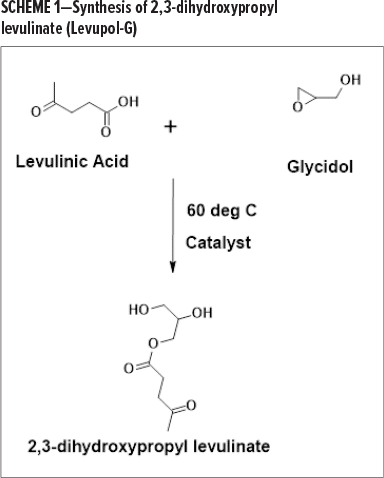
Any epoxide functional fluid with two or more oxirane groups can be utilized and a myriad of polyol grades can be obtained. For example, the reaction of levulinic acid with epoxidized soybean oil would result in soy polyol with crosslinkable levulinic ester in the final product, as shown in Scheme 2.
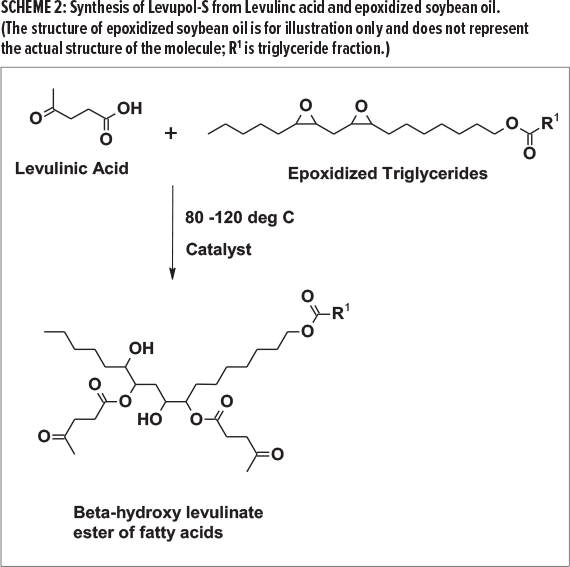
As shown in Scheme 1 and Scheme 2, the polyol has hydroxyl groups that are necessary to react with polyisocyanates to form the polyurethane backbone. It also contains the performance-enhancing keto group that reacts with crosslinkers and provide the high-performance features.
We adopted Green Chemistry Principles3 and the benefits of the polyol are: (a) 100% atom economical as there are no byproducts (b) it does not use any solvents, and (c) the product formed is highly pure, and no additional purification step is required.
The proprietary catalyst employed to make the polyol is highly effective for the epoxide ring opening with carboxylate nucleophiles. As a result, the ring opening reaction is fast, well-controlled, and produces the desirable product in quantitative yield.
There are no undesirable polyethers formed during the synthesis that would have otherwise adversely affected the product performance and cost. It was found the catalyst we used accelerated the product formation significantly compared to commercial control, as shown in Figure 1.
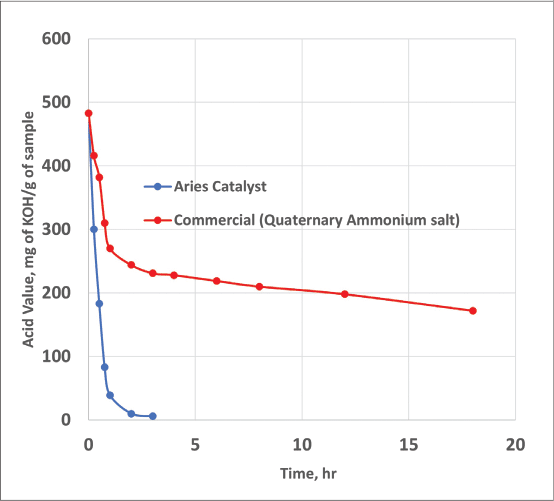
Figure 1