By Clifford K. Schoff, Schoff Associates
A friend recently wanted to know about pigment grinding, which made me realize that I had better begin this article by saying that we do not grind pigments, we disperse them. A bag of pigment contains clumps that must be separated into smaller clumps or individual particles. This is necessary in order to produce paint that provides good appearance on application and cure and does it economically. Pigment is expensive. Depending on the color, it may be the most expensive component in a paint.
The pigment dispersion process involves replacing air-solid interfaces in the dry powder with liquid-solid interfaces and separating the clumps of pigment particles so that they are dispersed in the liquid. The dispersed particles must be separated, or they will flocculate to form new clumps. The dispersant may be a polymer solution (possibly of a surfactant-like polymer), a surfactant solution or dispersion, a solvent or a combination of these. Wetting agents often are added to reduce interfacial tension and improve initial formation of the liquid-solid interfaces. The resulting dispersion commonly is called a pigment paste.
Pigment dispersion can be divided into three overlapping steps:
- Wetting—for good wetting, the dispersant must have a lower surface tension than that of the pigment. This enables the dispersant to displace air and water from pigment surfaces and penetrate pores, gaps, and channels between particles and adsorb onto them. Wetting agents often are used to facilitate this process. Another strategy is to modify the pigment surface to make it more wettable and, therefore, more easily dispersed. This usually is done by the pigment manufacturer by adsorbing a surfactant on the pigment to produce an “easy-disperse” grade.
- Deagglomeration—the wetted clumps are sheared by a blade, roller, sand, or beads, which break the pigment into smaller agglomerates or primary particles. Additional wetting occurs where particles have been freshly separated. Wetting of a pigment surface allows better stress transfer which improves shearing and provides more efficient breaking up of clumps.
- Stabilization—dispersants help with wetting and deagglomeration, but their main purpose is to stabilize the pigment particles. Dispersants adsorb on the particles and either impart a charge to the surface so that particles repel each other (charge
stabilization—waterborne pastes and paints) or build up an adsorbed layer to repel other particles (steric stabilization—
solventborne pastes and paints). The most important requirement is that the dispersant must stay on the pigment! This is not as simple as it may seem. The paste must be mixed with the portion of the formula called the letdown; whose solvents may strip the dispersant from the pigment. Paste-letdown temperature or viscosity differences also may destabilize the dispersion.
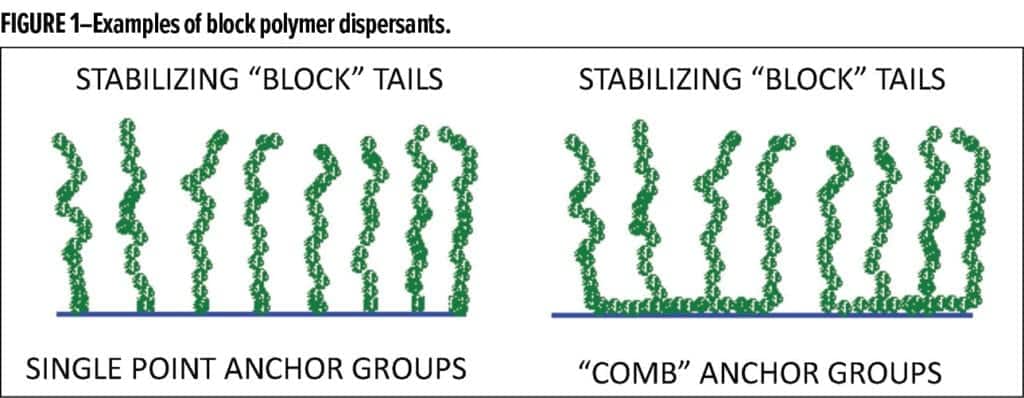
Dispersants contain anchoring groups that strongly adsorb on the pigment surface. Anchors are designed to be insoluble in the paint solvent package or have low solubility in it. Anchors have chains (also called tails) attached to them that that are soluble in the solvent (or the aqueous phase of a waterborne system) and have little or no affinity for the pigment surface. Dispersants used to be made by random polymerization that gave irregular structures which sometimes adsorbed on multiple pigment particles resulting in bridging flocculation. Most dispersants now contain block or graft copolymers with special anchoring groups and controlled structures. See Figure 1. Anchors usually are strong polar groups (which may be charged in waterborne systems) such as amines, carboxylic acids, sulfonates, phosphates, and ureas. Chains (tails) in solvent systems may be fatty groups C18H37 or longer, fatty polyesters or poly(isobutylene). In waterborne systems, they may be polyethylene oxide, block copolymers of ethylene oxide/propylene oxide, polyvinyl alcohol, or polyvinyl pyrrolidone.
Pigment dispersion requirements in the lab include good color development, a homogeneous paste, sufficient opacity or transparency and excellent stability as a paste and in a paint. The lab dispersion then must be scaled up to make paste batches in the paint plant. This often is difficult. The increase in volume changes shear and flow patterns and affects mixing. Velocities, shear stresses and shear rates often are less in the plant, sometimes much less, which can affect particle size and size distribution. Manufacturing requirements include consistent product, a definite end point (completion of dispersion), reasonable cost, ease of manufacturing, including the time required to produce a batch, the power requirement, equipment wear, ease of filtration, ease of clean-up, etc. The dispersion process rarely is optimized in either the lab or the plant, but that must not stop the technician or engineer from continuing to try to accomplish that objective.
I will cover the testing of pigment dispersions in the second part of this article.
CoatingsTech | Vol. 17, No. 5 | May 2020
