By Dr. Mark Vincent
Abstract
3,3’-Dichlorobiphenyl (PCB-11) is a polychlorinated biphenyl (PCB) congener that has attracted a lot of attention in recent years due to it being detected in the environment and its source not fully understood. PCB-11 was not a significant congener within the PCB Aroclor mixtures that were banned from production because they were persistent organic pollutants (POPs). Research to determine the source of PCB-11 in the environment has focused on its inadvertent presence in organic pigments, principally diarylide pigments. Other sources such as incineration, photochemical dechlorination of higher-level PCB congeners and other non-pigment containing products could also explain the higher than anticipated level of PCB-11 within the environment.
Introduction
Polychlorinated biphenyls (PCBs) have extremely good technical properties, such as chemical stability, low volatility, insulativity, and non-flammability. These outstanding properties meant they were applied to a wide number of industrial applications, such as coatings, inks, flame retardants, paints, electronic applications, heat transfer systems, and hydraulic fluids (Srogi, 2008), (Hongtao Shang, 2014) and were produced globally in quantities of at least 1.3 million tons (Breivik, 2002). PCBs were manufactured and solder under trade names such as Aroclor (Monsanto Industrial Chemicals Company, USA), Chlorextol (Allis-Chambers, USA), Clophen (Bayer, Germany), Delor (Chemko, former Czechoslovakia), Dykanol (Gornell Dubille, USA), Fenclor (Caffaro, Italy), Kanechlor (Kanegafuchi Chemical Company, Japan), Phenoclor and Pyralene (Prodelec, France) (IARC, 2015).
The United Nations Environment Program considers PCBs to be persistent organic pollutants (POPs), due to their ability to withstand degradation (United Nations, 2001). PCBs have been banned from manufacturing, processing, and distribution for many years, beginning in the late 1970s with regulations issued by the U.S. Environmental Protection Agency (EPA). Totally closed applications, such as in transformers, were still permitted; the EPA also recognized that PCBs could be created inadvertently in products such as pigments, setting a limit of PCB concentrations to be less than 50 parts per million (ppm) maximum and an average of 25 ppm (40 CFR Part 761). Since national bans were enforced, concentration of PCBs in the environment and humans have generally decreased (Vorkamp, K. R., 2012).
Despite their endurance, PCBs have been found to environmentally break down or degrade. The process varies, depending on the chemical makeup of the PCBs and where they are situated in the environment. The major degradation pathways are photo degradation, by sunlight, or by microorganism. In air, shallow water or surface soils, photochemical degradation is an important pathway, and microorganisms, such as bacteria, algae, and fungi, are important biodegradation pathways when PCBs are found in soil or sediments (National Ocean Service, n.d.).
The most frequently analyzed PCB congeners, which were present in industrial mixtures, are PCB-28, PCB-52, PCB-101, PCB-118, PCB-138, PCB-153, and PCB-180 (Vorkamp K. , 2016). In 2010, the EPA issued Method 1668C, which targeted the full list of 209 PCB congeners (EPA, 2010) and has generated more information and a focus on lesser-known or non-traditional PCB congeners.
One PCB congener receiving attention is PCB-11 (3,3’-dichlorobiphenyl), a non-traditional PCB that the use of Method 1668C has resulted in research to determine its presence and distribution in the environment (Vorkamp K. , 2016). Attempts have been made to determine how PCB-11 could enter the environment, with a significant focus on the previously known inadvertent generation when certain pigments are manufactured. In particular, diarylide pigments have been identified as being the sole or most significant source (Vorkamp K. , 2016), (Rodenburg L. G. J., 2015), (Hongtao Shang, 2014). Little attention has been dedicated to identifying other known sources of PCB-11. The objective of this study is to review published studies on PCB-11 that identify other sources contributing to its presence in the environment.
PCB-11 and its Presence in the Environment
PCB-11 is known to be present in the environment having been detected in the Lomonosofonna Glacier, Svalbard (Garmash O., 2013), the New York/New Jersey Harbor, and Delaware River (Rodenburg L. G. J., 2010), the Halifax Harbor in Canada (King, 2002), and the Spokane River in Washington (Wilks D., LimnoTech Inc., 2019). The presence of PCB-11 in the environment is not well understood. It was not a significant congener in the industrially produced products. Anderson et al. (Anderson, 1991) reported its detection in Aroclors 1016, 1221, 1232, and 1242, and Kim et al. (Kim, 2004) detected it in Kaneclor (KC 300, KC 400, KC 500, and KC 600). The highest mass was reported in Aroclor 1221 at 0.278 weight percent.
Garmash et al. analyzed all 209 PCB congeners in ice-core samples from the Lomonosofonna Glacier; the samples represented ice from the years 1975 to 2009 and snow from 2009 to 2010 (Garmash O., 2013). PCB-11 was found to be in all samples, representing 0.9-4.5% of the total PCB (Figure 1).
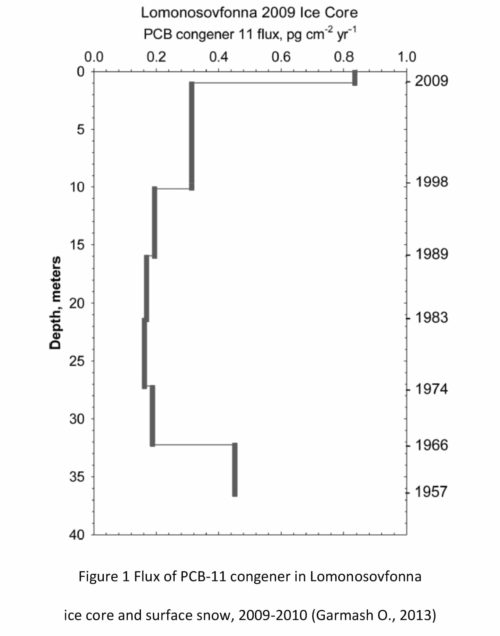
While the source of PCB-11 in the ice-core and snow samples was unknown, several postulations were made as to its presence. It was postulated to be presented due to thermal processes (smelting) found on the Kola Peninsula (Takasuga, Inoue, Ohi, Umetsu, & Ireland, 1994), or through its inadvertent generation in certain pigments, and from its generation in flue gas and ash as demonstrated by from a laboratory scale fluidized bed reactor from municipal solid waste incineration facilities in Sweden (Jansson, Lundin, & Grabic, 2011). Within these laboratory scale fluidized bed reactors that mimicked the municipal solid waste incineration facilities, PCB-11 was the dominant dichlorobiphenyl (DiCB) generated in all but one sample. The postulation that waste incineration was a significant generator of this previously uninvestigated PCB congener was further confirmed from a study that found flue gases from waste incineration plants in Japan were found to contain PCB-11 in amounts of 0.23-2.8% of total PCB (Kim, 2004). Although the concentration was less than what was detected in the Lomonosovfonna Glacier, Garmash considered it to be consistent with the range observed at Lomonosovfonna (0.9-4.5%). Garmash concluded more studies on PCBs originating from thermal processes (including incinerators) would be valuable to determine their extent as a source of PCB-11.
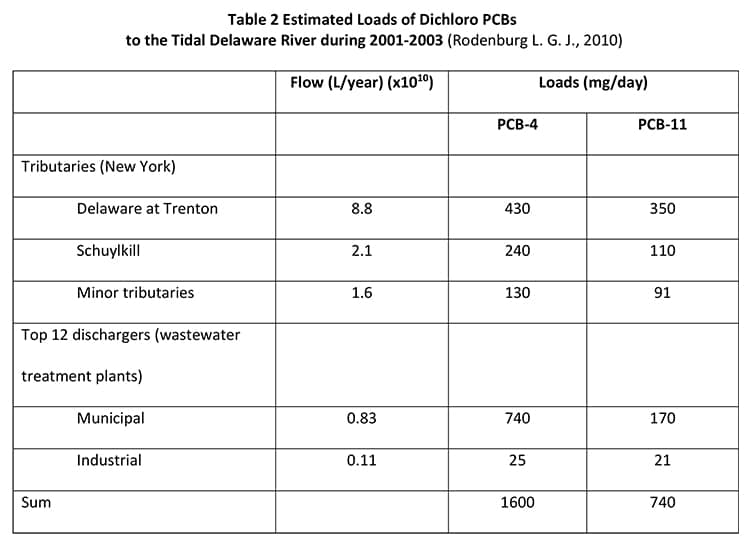
PCB-11 has been found in the New York/New Jersey Harbor and the Delaware River (Rodenburg, 2010), with the results displayed in Table 1 and Table 2.
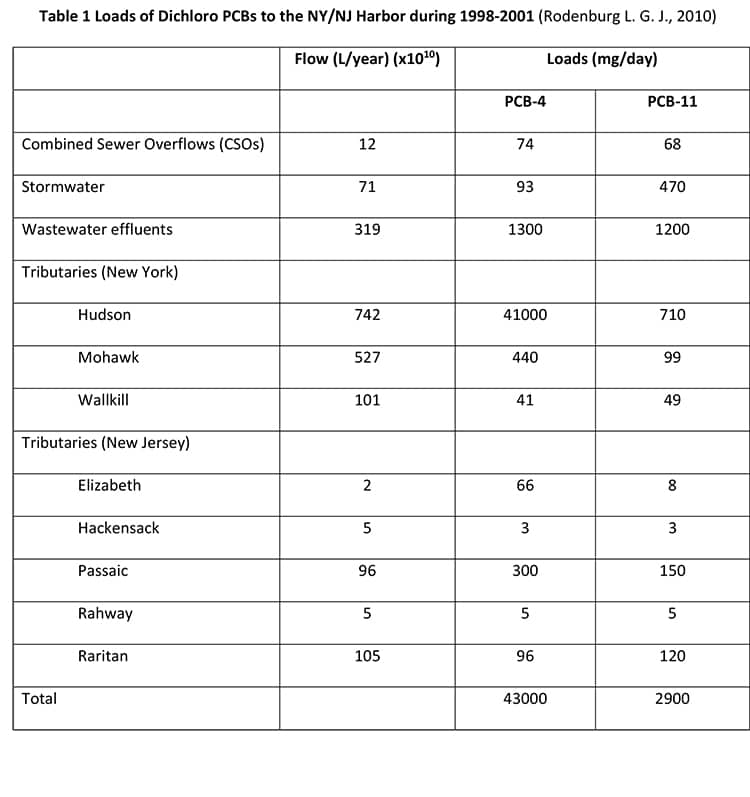
Rodenburg attributed the presence of PCB-11 in these watersheds to its inadvertent generation in the manufacture of diarylide pigments. The New York/New Jersey Harbor was downstream of pigment production. However, this was not true of the Delaware River, which had no known manufacturers of diarylide pigments. Despite this, the conclusion was the inadvertent generation of PCB-11 in diarylide pigment manufacture was the main source of PCB-11 in these systems. Rodenburg postulated that as the ratio of PCB-11 vs PCB-4, a sister DiCB, was higher downstream than upstream, and therefore the main source of PCB-11 within the two typical urban watersheds is not dechlorination of heavier congeners; but Rodenburg hypothesized that PCB-11 can be released from printed paper and cardboard materials that may be easily shredded and therefore can contribute to the particle phase PCB-11 burden in ambient waters which ultimately is released to the dissolved phase. Rodenburg’s analysis, however, did not consider the relative dechlorination rate of PCB-4 (faster) vs PCB-11 or other sources of PCB-11 that would account for its presence.
One significant error is that Rodenburg incorrectly estimated the global production of organic color pigments at a total of 250 million metric tons in 2006, whereas total global production was actually 250,000 metric tons (Savastano, 2007). Rodenburg more accurately estimated total diarylide pigment production at 40% of total organic color pigment production. Using an estimated PCB-11 content of 38ppb, Rodenburg estimated total inadvertent generation of PCB-11 in diarylide pigments at 1.5t in 2006. Given the overestimate in global pigment production by a factor of 1000 (250 million vs 250,000 metric tons), this would mean the estimated global PCB-11 inadvertent generation in diarylide pigments was actually 0.15t, instead of 1.5t, in 2006. Although its presence as an inadvertent byproduct in the manufacture of diarylide pigments cannot be ignored, other sources of PCB-11 must also be contributing to the environmental mass balance.
Rodenburg determined that dechlorination of higher-level PCB congeners cannot be a significant source of PCB-11 by examining the ratios of PCB-11 to PCB-4 (2,2’-dichlorobiphenyl) upstream and downstream of the New York-New Jersey Harbor. Upstream of the New York-New Jersey Harbor, there were lower levels of PCB-11 relative to higher amounts of a sister lower-congener PCB-4. However, downstream the ratio of PCB-11 increased compared to PCB-4. Based on this, Rodenburg concluded that dechlorination of higher-level PCB congeners cannot be the source of PCB-11 within the New York-New Jersey Harbor and therefore its presence in pigments must be the only or most likely source. This conclusion requires further investigation as it is known that the source and presence of PCBs in the environment is much more complex. For example, PCB-11 has been shown to be slower to photochemically degrade than other PCBs (Yao Y., 1997) and PCB-11 has been detected in products other than pigments (City of Spokane, 2015). It is not possible to conclude that the only source of PCB-11 is from pigments. New data, such as that generated by the City of Spokane (City of Spokane, 2015), suggest other sources of PCB-11 are present in the New York-New Jersey Harbor.
In a different study (Rodenburg, 2014), Rodenburg claimed that a mass balance can be used to prove that pigments present in the Delaware River Basin account for the PCB-11 measured in the Delaware River water. The balance between pigments and river water, however, required an assumed concentration of 125 ppm of PCB-11, which is the maximum amount of inadvertent PCBs allowed under TSCA, for all diarylide pigment present in the Delaware River Basin. This is an inaccurate over-assumption also, given the earlier paper (Rodenburg L. G. J., 2010) estimated a PCB content in diarylide pigments of 38 ppb and also requires the assumption all pigments contain the maximum legally permitted amount.
Further investigation is needed to provide a more complete picture of the presence of PCB-11 within the New York-New Jersey Harbor and the Delaware River. For example, incineration taking place along or upwind of the watersheds can certainly be a source and knowing the PCB profile in these emissions would be very revealing (Jansson, Lundin, & Grabic, 2011). It is known that PCB-11 is a stable dichloro congener compared to its sister PCB congeners during photochemical breakdown (Yao Y., 1997). As such, its increased ratio or presence in downstream samples could be or would be anticipated.
Research conducted by the City of Spokane (City of Spokane, 2015) shows other sources of PCB-11 exist. Such sources included motor oil (City of Spokane, 2015), road dust suppressant (City of Spokane, 2015), laundry detergent (City of Spokane, 2015), among others, and these, along with other unknown sources, require further investigation. Such sources are used and produced in higher volumes, and, given their use and proximity to sewers, have a higher likelihood to be discharged to rivers and other watersheds.
PCB-11 and other PCB congeners have been detected in the Spokane River, Washington (Wilks D., LimnoTech Inc., 2019) (Spokane County, Washington, 8-9 October 2019). Figures 2 and 3 show the range of PCB congeners detected.
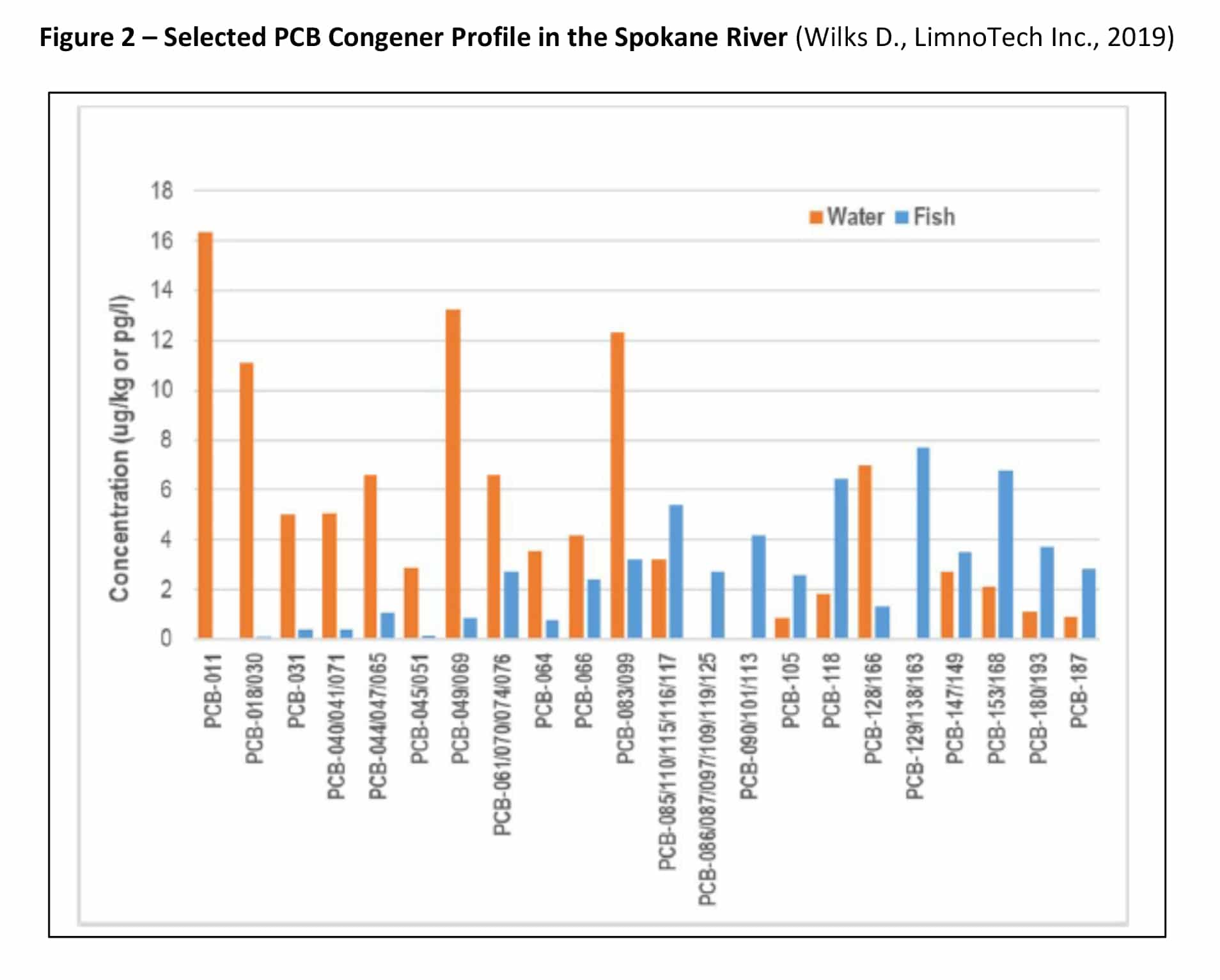
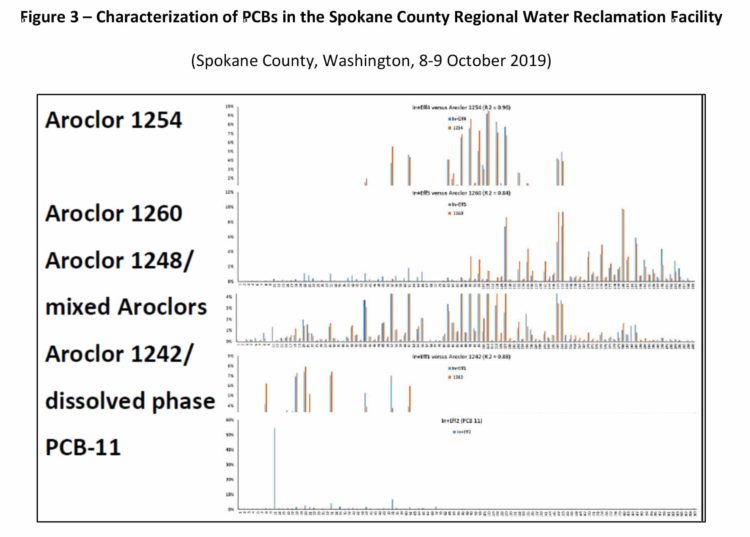
It has been proposed that the sole source of PCB-11 in the Spokane River originates from printed matter containing diarylide pigments that contain inadvertent concentrations of PCB-11 (Rodenburg, 2015). Specifically, the contention is that the PCB-11 Spokane River concentration originates from discarded/landfilled printed matter or from paper recycling operations discharging to the Spokane River. However, the presence of PCB-11 in wastewater from the sewer treatment facilities and stormwater in concentrations exceeding those found in effluent from paper recycling facilities provides a strong indication that other sources of PCB-11 exist, such as incineration, photolysis and its inadvertent presence in other non-pigment-containing products. The inadvertent generation of PCB-11 in pigments and potential subsequent contribution of PCB-11 to the environment is well documented (Hongtao Shang, 2014) (Rodenburg L. G. J., 2010) (Rodenburg L. G. J., 2015) (Vorkamp K. , 2016), other contribution sources are less known. These new but important sources and pathways will be reviewed in the following sections.
Incineration
Potential sources of non-Aroclor PCBs are municipal solid-waste incinerators (MSWIs). It has been demonstrated that PCB-11 and other PCBs can be released either by direct emission from PCB-containing combustion materials or through formation from carbon and chlorine precursors. Measurement of stack gases from MSWIs in South Korea, detected trace amounts of PCB-11, although the contribution to total PCBs was minimal (Ikonomou M.G., 2002). In Sweden, municipal waste was found to generate flue gases and ashes containing PCB-11 as high as 7% of total PCBs during combustion between 200 and 450 °C in a laboratory-scale waste incinerator (Jansson, Lundin, & Grabic, 2011). Mono- and di-chlorinated PCBs were dominant in the flue gases, with PCB-11 among the most abundant PCBs. This was not unexpected as this feature is known to be a favored formation of meta and para substituted congeners in such situations.
In Japan, PCB emissions from multiple conditions of waste incinerations were investigated (Kim, 2004). Variables analyzed included types of incinerator, combustion temperatures (from 740 to 920 °C), and the nature of waste materials. PCB-11 was detected in all flue gas samples. In two of eight samples, significantly higher concentrations, as well as certain dioxin-like PCBs, were measured. This result was found to occur when lower combustion temperatures or waste plastic in the combustion materials was present. It is interesting to note that Ogura et al. (Ogura I., 2005) estimated that incineration is roughly equal in importance as compared to legacy sources as a source of dioxin-like PCBs in Japan, although PCB-11 was not specifically consider PCB-11.
Within the United States, the PCB profile from incinerators has not been well documented, and there remains a knowledge gap that needs to be filled to better understand its contribution of PCB-11 and other PCB congeners to the environment.
The findings by Jansson (Jansson, Lundin, & Grabic, 2011) in Sweden and Ogura (Ogura I., 2005) and Japan strongly suggest that incineration in the locals area would be a source PCB-11 in the New Jersey/New York Harbor and the Spokane River. Such sources of incinerator flue gasses could explain why PCB-11 content increases in water facilities such as the Spokane County Regional Water Reclamation Facility during storm events causing it to be washed down from the atmosphere or after deposition. (Spokane County, Washington, 8-9 October 2019)
Photochemical Dechlorination/Photolysis
Photolysis is a known method for the degradation of PCBs. In 1972, Hutzinger et al. (Hutzinger O., 1972) reported a time study of 2,2’,5,5’-tetrachlorobiphenyl (PCB-52) decomposition by irradiating in hexane and under aqueous conditions. It was shown that PCB-52 decomposes over time to lower-level PCBs, of which PCB-11 would be one. Gas chromatograms were analyzed demonstrating the breakdown of PCB-52 to lower-level PCBs, but no attempts were made to characterize and identify these PCB congeners. Aroclor 1254, a previously produced commercial product, containing a mixture of PCBs was also irradiated. It was again demonstrated that the higher-level PCBs within Aroclor 1254 slowly degraded over time, with lower-level PCB congeners increasing. It was concluded that the photolysis of PCBs reveals several degradative reactions which occur on irradiation in sunlight and in laboratory conditions. Irradiation of PCB-52 in aqueous media indicated complex degradation pathways, including dechlorination, formation of polymers, carboxylic products, as well as hydroxylation.
In 1973, Herring et al. (Herring, 1973) reported an irradiation study of Aroclor 1254 in hexane, water, and benzene. Gas chromatograms were completed pre- and post-irradiation; however, as with Hutzinger et al. (Hutzinger O., 1972), no attempts were made to characterize the breakdown products. Herring et al. found that PCBs degraded fastest in hexane, then water, and slowest in benzene. PCBs also do not photo degrade at the same rate a conclusion that is important when assessing PCB congeners and their degradation rates in the environment.
In 1974, Ruzo et al. (Ruzo L., 1974) studied a variety of tetrachlorobiphenyl congeners and their degradation products under UV irradiation in cyclohexane. Their study included 3,3’,4,4’-tetrachlorobiphenyl (PCB-77), 2,2’4,4’-tetrachlorobiphenyl (PCB-47), 3,3’,5,5’-tetrachlorobiphenyl (PCB-80), 2,2’,3,3’-tetrachlorobiphenyl (PCB-40), 2,2’,5,5’-tetrachlorobiphenyl (PCB-52) and 2,2’,6,6’-tetrachlorobiphenyl (PCB-54). It was found that PCB-40 photodegraded to 2,3,3’-trichlorobiphenyl (PCB-20), 2,2’,3-trichlorobiphenyl (PCB-16) and PCB-11 as the major products. PCB-52 also produced PCB-11, photodegrading to 2,5,3’-trichlorobiphenyl (PCB-26), PCB-11 and 3-chlorobiphenyl (PCB-2) as the major products. This was the first study that clearly identified (some) of the reductive dechlorination products from photolysis of higher-level PCB congeners. Within this study both PCB-40 and PCB-52 were demonstrated to photodegrade to PCB-11. This study is important as PCB-40 has been reported to be present at relatively high levels in the environment, such as in the Spokane River watershed (Wilks D., LimnoTech Inc., 2019) and both PCB-40 and 52 have been reported to be present in the Spokane County Regional Water Reclamation Facility (Spokane County, Washington, 8-9 October 2019). PCB-40 and 52 are also known congeners to be present in commercialized Aroclor products. Based on this finding, photodegradation of PCB-40 and 52 is a source of PCB-11 in the environment.
In 1992, Harari et al. (Hawari J., 1992) investigated the sensitized photolysis of Aroclor 1254, using phenothiazine (PT) as the sensitizer. Aroclor 1254 was completely dechlorinated under lab irradiation within 1 hour to biphenyl (BP), in the presence of PT in alkaline 2-propanol. Research carried out under sunlight, determined that complete photolysis did not occur, but low-level PCB congeners such as 3,3’-dichlorobiphenyl (PCB-11), 2,3’,5-trichlorobiphenyl (PCB-26), and PCB congeners with average chlorine content of 3-4 Cl atoms/PCB molecule were detected. The amount of PCB-11 etc. detected was not reported; however, it is evident that Aroclor 1254 photodegrades to PCB-11. Within the known detections in the environment, Aroclor 1254 has been reported to be a major PCB constituent in the Spokane County Regional Water Reclamation Facility (Spokane County, Washington, 8-9 October 2019). Within this same facility the breakdown products PCB-11 and PCB-26 have been detected suggesting that photolysis of Aroclor 1254 is contributing to the PCB-11 loading in the environment.
In 1997, Yao et al. (Yao Y., 1997) reported their research of the photochemical degradation of 3,3’4,4’-tetrachlorobiphenyl (PCB-77) which demonstrated the stepwise dechlorination of PCB-77 to its breakdown products as follows:
3,3’4,4’-tetrachlorobiphenyl (PCB-77) →
3,3’4-trichlorobiphenyl (PCB-35) + 3,4,4’-trichlorobiphenyl (PCB-37) →
3,3’-dichlorobiphenyl (PCB-11) + 3,4’-dichlorobiphenyl (PCB-13) +4,4’-dichlorobiphenyl (PCB-15) →
3-chlorobiphenyl (PCB-2) +4-chlorobiphenyl (PCB-3) → Biphenyl
This degradation is demonstrated in Figure 4.
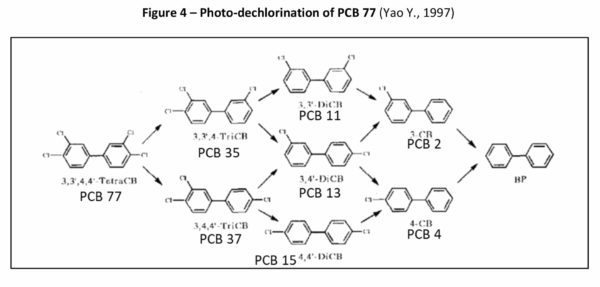
Yao et al.’s study showed that at a point in the irradiation process, PCB-11 was the highest concentration of all the congeners that formed and did not completely disappear at the end of the experiment after 4 hours of irradiation time; 5% of PCB-11, 5% of PCB-2 and 13% of biphenyl remained. In addition to identifying the congeners formed, the rate of photolysis for each congener was also determined. The rate of photolysis for each congener was found to be in the order of PCB-37=PCB-35>PCB-77>PCB-3>PCB-2>PCB-13>PCB-15>PCB-11. PCB-11 was the slowest to photodechlorinate. This supports the hypothesis presented by Herring et al. in 1973 that PCB-11 is a more stable PCB compared to other PCB congeners, at least to photodegradation.
While PCB 77 is a minor contaminant in the environment such as the Spokane River watershed (Wilks D., LimnoTech Inc., 2019) and the Spokane County Regional Water Reclamation Facility (Spokane County, Washington, 8-9 October 2019), Yao et al.’s research supports that high level PCB congeners do photodegrade to lower level PCB congeners such as PCB-11 and that PCB-11 is a stable congener of the breakdown products and would be expected to accumulate at higher rates in the environment as a result.
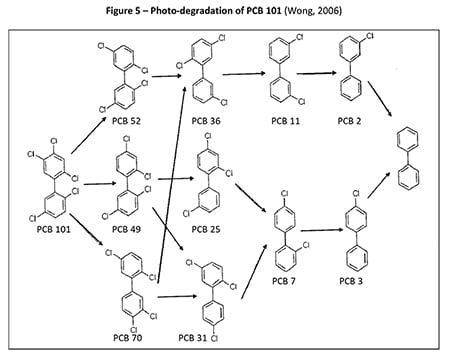
In 2006, Wong et al. (Wong, 2006) studied the photolysis of various PCB congeners, including 2,4,4’-trichlorobiphenyl (PCB-28), 2,2’,5,5’-tetrachlorobiphenyl (PCB-52), 2,2’,4,5,5’-pentachlorobiphenyl (PCB-101), 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB-153) and 2,2’,3,4,4’,5,5’-heptachlorobiphenyl (PCB-180). The authors identified that the intermediates produced were mainly less chlorinated congeners as reported in previous studies (Yao Y., 1997). The degradation pathway for PCB-101 was provided where PCB-11 was an identified intermediate dechlorinated product as shown in Figure 5.
There are 209 PCB congeners, all of which are present in the environment and watersheds to greater or lesser extents. Within the Spokane River Watershed (Wilks D., LimnoTech Inc., 2019) and the Spokane County Regional Water Reclamation Facility (Wilks D., LimnoTech Inc., 2019) several congeners have been identified with the potential to photochemically dechlorinate to PCB-11. These congeners are presented in Table 3.
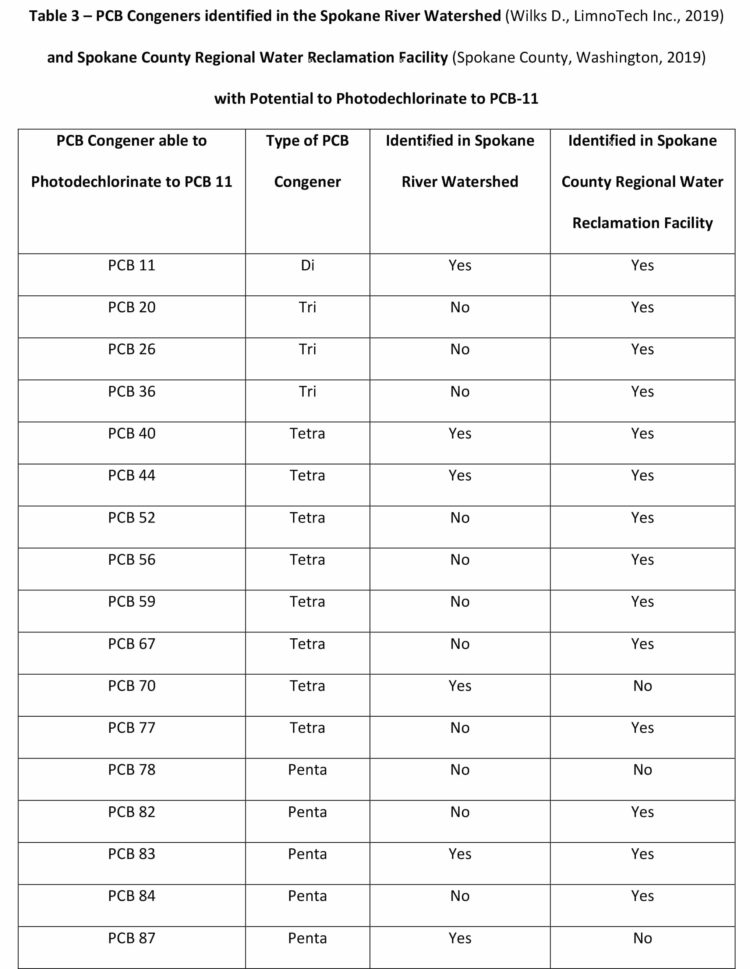
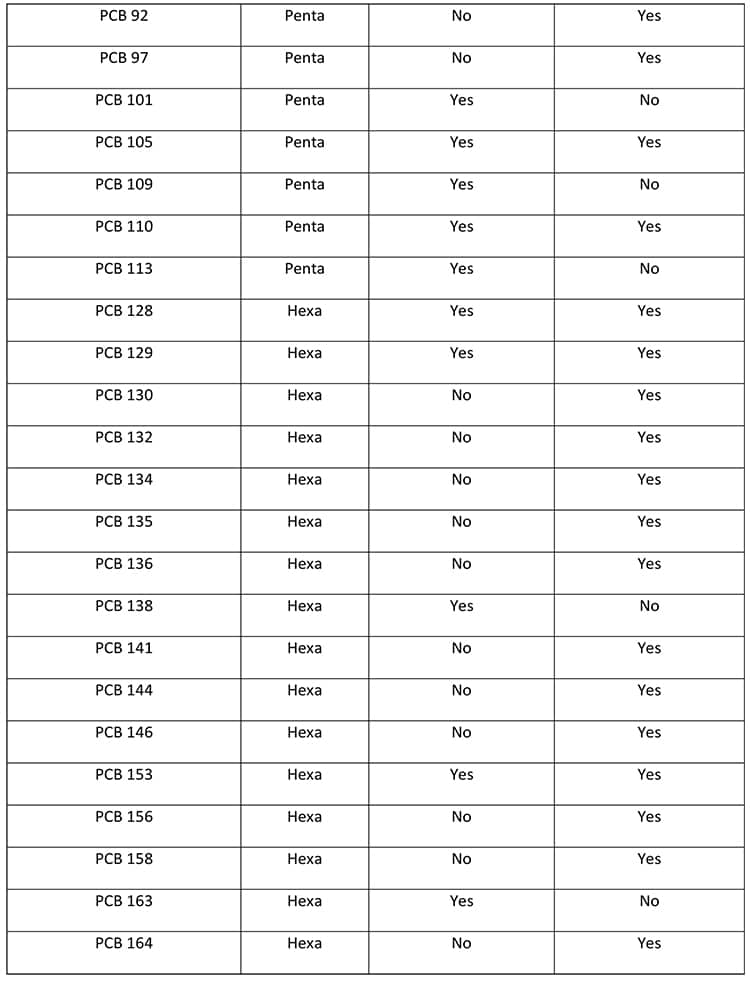

There are a total of 65 PCB congeners that have been identified in the Spokane River Watershed or the Spokane County Regional Water Reclamation Facility that have the potential to photochemically dechlorinate to PCB-11. Figure 6 demonstrates how higher-level PCBs congeners “funnel” or “concentrate” to the more photochemically stable (Yao Y., 1997) and environmentally accumulating PCB-11. This potentially demonstrates why PCB-11 occurs in the environment, such as the New Jersey/New York Harbor, the Spokane River Watershed, and the Spokane County Regional Water Reclamation Facility, at higher levels than expected.
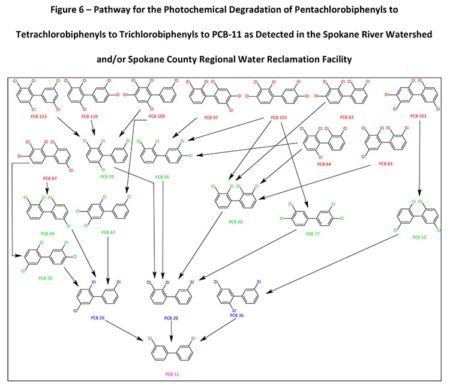
Figure 6 only represents the photodegradation pathway from the pentachlorobiphenyls congeners. If the hexa, hepta, octa, nona, and decachlorobiphenyls are considered (as in Table 1), the level of congeners potentially photodegrading in the environment to PCB-11 increases significantly.
Within Figure 6, there are 10 pentachlorobiphenyl congeners that potentially would photodegrade to eight tetrachlorobiphenyls which sequentially photodegrade to three trichlorobiphenyls that ultimately degrade to one PCB-11 congener. Figure 6 depicts the PCB congeners that have been detected and presented in the Spokane River Watershed or the Spokane County Regional Water Reclamation Facility test data. Of the 10 pentachlorobiphenyl congeners depicted in Figure 6, nine congeners (PCB-82, 83, 84, 87, 97, 101, 105, 110, 113) have been detected at significant levels. Of the eight tetrachlorobiphenyl congeners, three congeners (PCB-40, 44, 70) and of the three trichlorobiphenyl congeners identified, two congeners (PCB-20, 26) have been detected at significant levels. The literature on the photochemical dechlorination and demonstrated in Figure 4, provides strong evidence to suggest why PCB-11 is being detected at higher-than-expected levels in the environment, such as the Spokane River.
Detection of PCB-11 in Other Products
A study on PCBs conducted by the City of Spokane in Municipal Products (City of Spokane, 2015), detected PCBs in 39 of 41 product samples, with a wide range of congener patterns. The most detected congeners were the coeluting congeners PCB-52/69 (detected in 30 of the samples), followed by PCB-11 and PCB-28 (detected in 25 of samples).
The historically uninvestigated low-level PCB congener, PCB-11, was detected in 25 commercial products. Interestingly, only six of the products contained organic color pigments. 76% (19) of the products found to contain PCB-11, did not contain organic color pigments. Despite this, the City of Spokane incorrectly concluded that pigments are likely the common source of the PCB-11 detected. It is very clear there are other non-pigment-based sources of inadvertently generated PCB-11 to the environment.
PCB-11 was detected in non-pigment-containing products such as synthetic and used motor oil (City of Spokane, 2015) (Figure 7), road dust suppressants such as EADA (City of Spokane, 2015) (Figure 8) and magnesium chloride (City of Spokane, 2015) (Figure 9), asphalt release agent (City of Spokane, 2015) (Figure 10), asphalt crack sealer (City of Spokane, 2015) (Figure 11, PCB-11 was detected at >4% of all PCB congeners in this sample), laundry soap (City of Spokane, 2015) (Figure 12) among many others. It is clear these products do not contain organic color pigments, such as diarylide pigments, and therefore allows for the conclusion that pigments are not the only or most significant contributor of PCB-11 in the environment. It would be anticipated that products such as motor oil, road dust suppressants, asphalt release agents or laundry soap would be greater contributors to PCB-11 detection in the environment.
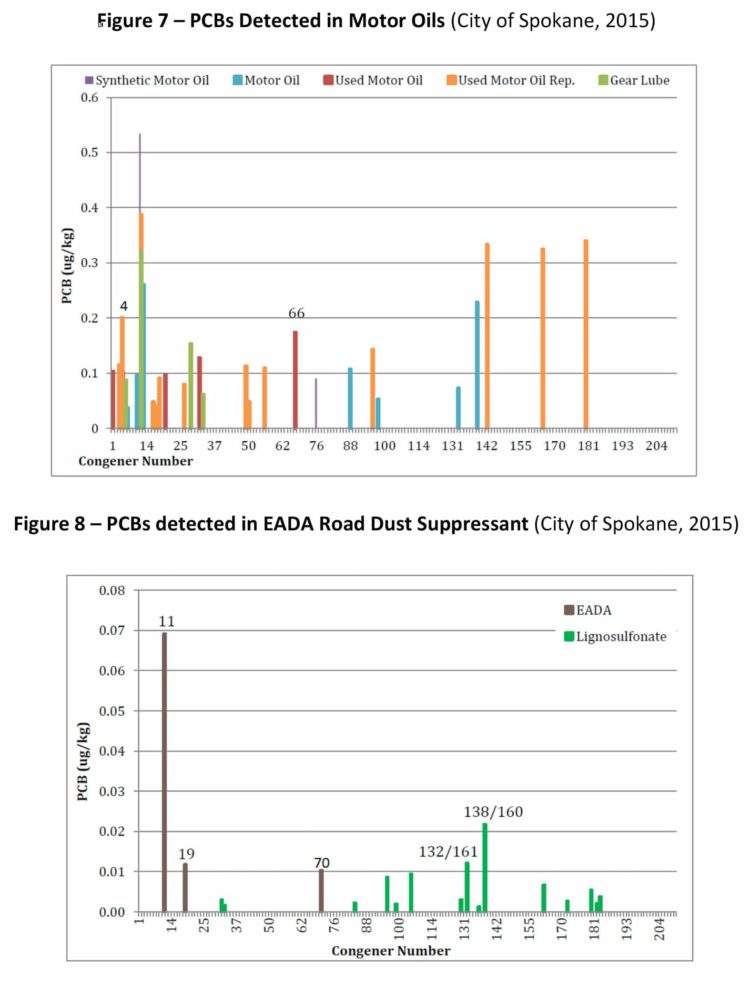
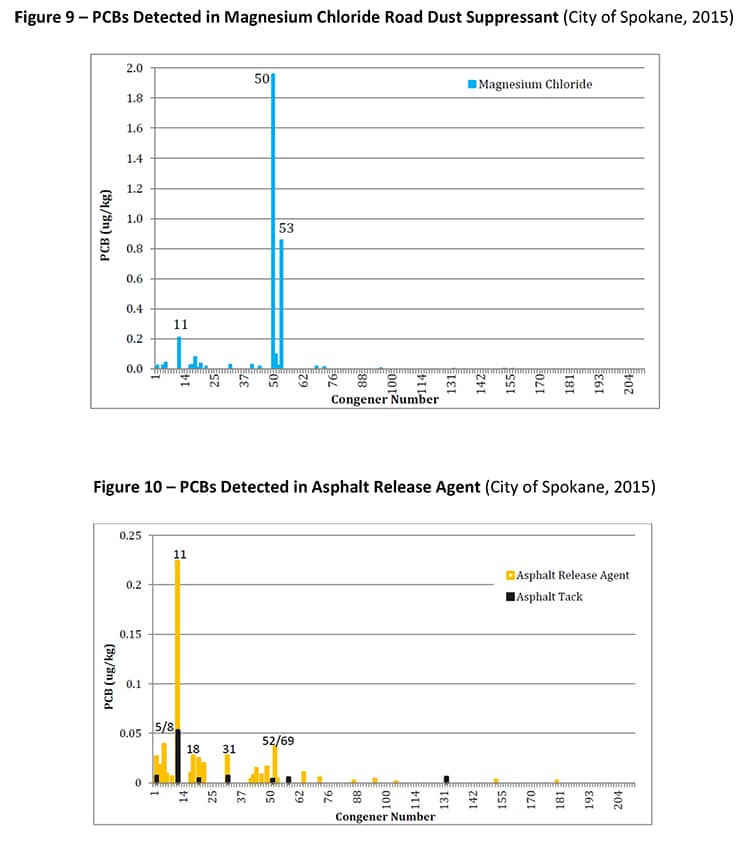
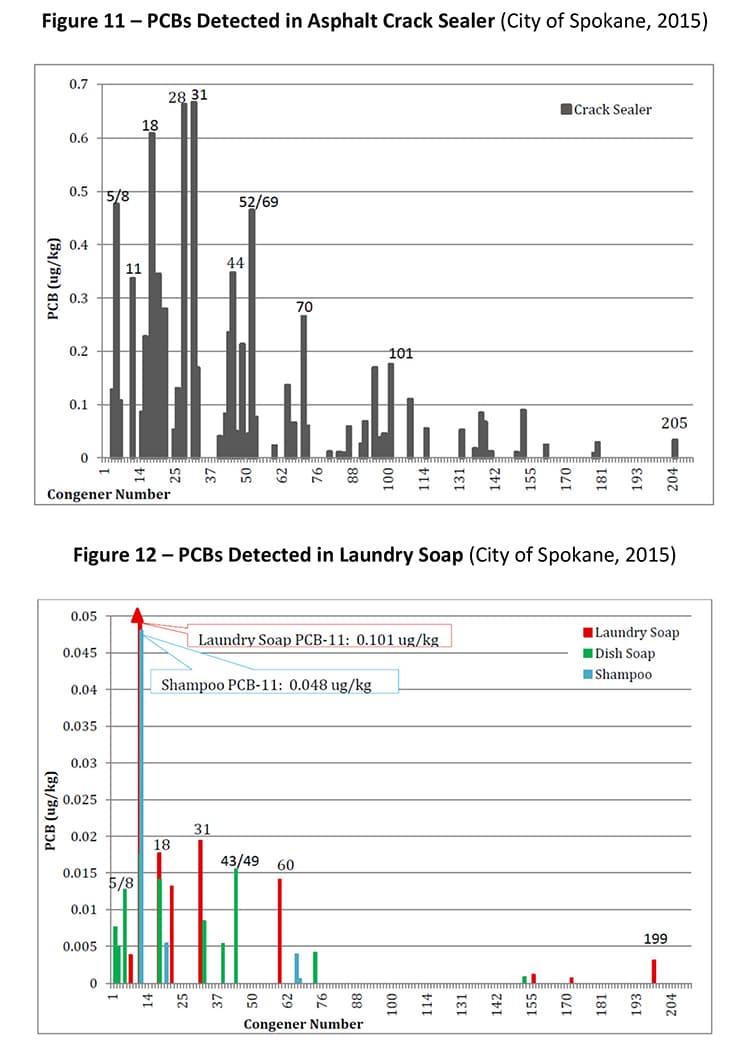
Another potentially significant source of PCB-11 is from the degradation of PCB-52 in these products, which was found to be one of the more frequent congeners present in this study. The detection of PCB-52 in products must be noted and included as a source as it has been shown to photochemically degrade to PCB-11 (Wong, 2006).
Conclusions
PCB-11 has been detected in the environment at several locations. While its presence as an inadvertently generated PCB in pigments is known, it is not the only source. Clear evidence exists that PCB-11 can be introduced into the environment from incinerator flue gases, by photochemical breakdown of higher-level PCB congeners, and in other non-pigment-containing products such as motor oil, road-dust suppressant, and laundry detergent. The full breadth of other sources of PCB-11 is not known and further research is required to better understand and map these sources and to understand how it enters the environment.
Until its environmental profile is better understood, remedial actions focused on a single and nonprimary source, such as diarylide pigments, will be ineffective.
About the Author
Dr. Mark Vincent, PhD, Mississauga, Ontario, Canada. markvincent@chromasc.com
Acknowledgments
This study was supported by the Color Pigments Manufacturers Association. “PCB-11 and its Presence in the Environment” was first published in Ink World in November 2020, following Dr. Vincent’s presentation at the National Association of Printing Ink Manufacturers’ 2020 virtual NPIRI Technical Conference.
References
Anderson, J. W. (1991). Determination of congeners of polychlorinated biphenyls in reference materials. J. High Resolut. Chromatogr., 14, 369-272. Retrieved from https://onlinelibrary.wiley.com/doi/abs/10.1002/jhrc.1240140603
Breivik, K. S. (2002). Towards a global historical emission inventory for selected PCB congeners — a mass balance approach: 1. Global production and consumption. Sci. Total Environ., 290 (1-3), 181-198. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0048969701010750
Bunce N., K. Y. (1978). An assessment of the Impact of Solar Degradation. Chemosphere, 155-164.
City of Spokane, W. M. (2015). PCBs in Municipal Products. Spokane. Retrieved from https://www.spokanecounty.org/DocumentCenter/View/3407/Study—PCBs-in-Municipal-Products-PDF?bidId=
Garmash O., H. M. (2013). Deposition History of Polychlorinated Biphenyls to the Lomonosovfonna Glacier, Svalbard: A 209 Congener Analysis. Environmental Science & Technology, 12064-12072. Retrieved from https://pubs.acs.org/doi/abs/10.1021/es402430t
Hawari J., D. A. (1992). Sensitized Photolysis of Polychlorinatedbiphenyls in Alkaline 2-Propanol: Dechlorination of Arocloro 1254 in Soil Samples by Solar Radiation. Environ. Sci. Technol., 26, 2022-2027. Retrieved from https://pubs.acs.org/doi/abs/10.1021/es00034a022
Herring, E. H. (1973). UV Irradiation of Aroclor 1254. Bulletin of Environmental Contamination & Toxicology, 8, 153-157. Retrieved from https://pubmed.ncbi.nlm.nih.gov/4628539/
Hongtao Shang, Y. L. (2014). The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,30-dichlorobiphenyl (PCB 11). Chemosphere, 98, 44-50. Retrieved from https://pubmed.ncbi.nlm.nih.gov/24231041/
Hutzinger O., S. S. (1972). Photochemical Degradation of Chlorobiphenyls (PCBs),. Environmental Health Perspectives, 15-20. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1474860/
IARC. (2015). Polychlorinated and polybrominated biphenyls. IARC Monographs on the Evaluation of Carcinogenic Risks for Humans. (W. H. International Agency For Research on Cancer, Ed.) 107, ISBN 978 92 832 0173 1. Retrieved from https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Polychlorinated-Biphenyls-And-Polybrominated-Biphenyls-2015
Ikonomou M.G., S. P. (2002). PCB levels and congener patterns from Korean municipal waste incinerator stack emissions. Chemosphere, 205. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0045653502001029
Jansson, S., Lundin, L., & Grabic, R. (2011). Characterisation and fingerprinting of PCBs in flue gas and ash from waste incineration and in technical mixtures. Chemoshphere, 509-515. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0045653511009593
Kim, K. S. (2004). Detailed PCB congener patterns in incinerator flue gas and commercial PCB formulations (Kanechlor). Chemosphere, 55, 539-553. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0045653503011998
King, T. Y. (2002). Tracing the source of 3,3′-dichlorobiphenyl found in samples collected in an around Halifax Harbour. Mar. Pollut. Bull., 44, 590-596. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0025326X01002892
National Ocean Service. (n.d.). What are PCBs? Retrieved from https://oceanservice.noaa.gov/facts/pcbs.html
Ogura, I. (2005). Quantitative identification of sources of dioxin‐like polychlorinated biphenyls in sediments by a factor analysis model and a chemical mass balance model combined with monte carlo techniques. Environ. Toxicol. Chem., 277. Retrieved from https://setac.onlinelibrary.wiley.com/doi/abs/10.1897/04-0221R.1
Rodenburg, L. (2010). Evidence for Unique and Ubiquitous Environmental Sources of 3,3′-Dichlorobiphenyl (PCB 11). Environ. Sci. Technol., 44, 2816-2821. Retrieved from https://pubs.acs.org/doi/10.1021/es901155h
Rodenburg, L. (2015). Polychlorinated biphenyls in pigments: inadvertent production and environmental significance. Coloration Technology, 131, 353-369. Retrieved from https://onlinelibrary.wiley.com/doi/abs/10.1111/cote.12167
Rodenburg, L. (2014). Global Distribution and Local Impacts of inadvertently generated PCBs in pigments. Env. Sci & Tech, 48, 8573-8580. Retrieved from https://pubs.acs.org/doi/10.1021/es502291b
Ruzo L., (1974). Photochemistry of Bioactive Compounds. Photochemical Processes of Polychlorinated Biphenyls. Journal of the American Chemical Society, 96, 3809-3813. Retrieved from https://pubs.acs.org/doi/abs/10.1021/ja00819a016
Savastano, D. (2007, March). The Pigment Report. Ink World. Retrieved from https://www.inkworldmagazine.com/contents/view_features/2007-03-13/the-pigment-report-105349/
Spokane County, Washington. (8-9 October 2019). Characterization of PCBs in the Spokane County Regional Water Reclamation Facility. Presentation from the Inadvertently Produced PCBs in Inks and Pigments Workshop. Retrieved from https://srrttf.org/wp-content/uploads/2019/10/6.-Sumner_InadvertPCBConf_Oct2019_rev3.pdf
Srogi, K. (2008). Levels and congener distributions of PCDDs, PCDFs and dioxin like PCBs in envionmental and human samples; a review. Environ. Chem. Lett., 6, 1-28. Retrieved from https://link.springer.com/article/10.1007/s10311-007-0105-2
Takasuga, T., Inoue, T., Ohi, E., Umetsu, N., & Ireland, P. T. (1994). Characterisation of PCBs formed during thermal processes. Organohalogen Compd., 173-176. Retrieved from https://pubs.acs.org/doi/abs/10.1021/es402430t
United Nations, E. P. (2001). Final Act of the Conference of Plenipotentiaries on The Stockholm Convention on Persistent Organic Pollutants. 44. Geneva, Switzerland. Retrieved from https://www.wipo.int/edocs/lexdocs/treaties/en/unep-pop/trt_unep_pop_2.pdf
United States EPA. (2010). Method 1668C: chlorinated biphenyl congeners in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. Retrieved from https://19january2017snapshot.epa.gov/sites/production/files/2015-09/documents/method_1668c_2010.pdf
Vorkamp, K. (2016). An overlooked environmental issue? A review of the inadvertent formation of PCB-11 and other PCB congeners and their occurrence in consumer products and in the environment. Science of the Total Environment, 541, 1463-1476. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26490526/
Vorkamp, K. R. (2012). Determination of Polychlorinated Dibenzo-pdioxins, Polychlorinated Dibenzofurans, and Dioxin-like Polychlorinated Biphenyls (PCBs) in Sediment and Biota. ICES Techniques in Marine Environmental Sciences, 50, 24. Retrieved from https://www.ices.dk/sites/pub/Publication%20Reports/Techniques%20in%20Marine%20Environmental%20Sciences%20(TIMES)/TIMES50.pdf
Washington State Department of Ecology. (2014). Polychlorinated Biphenyls (PCBs) in General Consumer Products. Publication No. 14-04-035. Retrieved from https://fortress.wa.gov/ecy/publications/documents/1404035.pdf
Wilks D., LimnoTech Inc. (2019). PCBs in our Watershed, PCBs in Products, and TSCA Exclusions: Putting It All Together. Presentation from Inadvertently Produced PCBs in Inks and Pigments Workshop, 8-9 October 2019. Retrieved from https://srrttf.org/wp-content/uploads/2019/10/5.-iPCB_Dilks10082019.pdf
Wong, K. W. (2006). Degradation of Polychlorinated Biphenyls by UV-Catalysed Photolysis. Human and Ecological Risk Assessment, 259-269. Retrieved from https://www.researchgate.net/publication/240520105_Degradation_of_Polychlorinated_Biphenyls_by_UV-Catalyzed_Photolysis
Yao Y., K. K. (1997). Photodechlorination Pathways of Non-Ortho Substituted PCBs by Ultraviolet Irradiation in Alkaline 2-Propanol. Bull. Environ. Contam. Toxicol., 59, 238-245. Retrieved from https://link.springer.com/article/10.1007/s001289900470
