By , , and
Introduction
The coatings industry has undergone significant transformations in recent years due to the replacement of organic solvent-based coatings by waterborne emulsion latex polymer coatings. Waterborne technology has generated profound impact in industry and everyday life, producing great environmental and health benefits. The prevalence of volatile organic compounds (VOCs) in coatings has been greatly reduced, from 700 g/L in the 1940s to ∼ 50 g/L in the 2010s.1 Although waterborne coatings offer considerable benefits, challenges remain in areas such as water resistance, stability, film formation, and surface hardness, which can affect their overall performance and application.
These challenges are primarily caused by the conflicting requirements for desired properties before and after the coating dries. To maintain a stable dispersion before application, latex particles should be fully dispersible in water, i.e., hydrophilic. However, after the coating dries, water repellence, or hydrophobicity, is required. Thus, a solution that can maintain predominantly hydrophilic dispersion while rendering a hydrophobic surface after drying is necessary to make waterborne coating technology more versatile. In addition, a durable coating film demands excellent adhesion on the substrate surface, while showing good hardness (low tackiness) at the coating-air interface. This means it is beneficial to possess different or even opposite properties on the two sides of the coating films.
One approach to combine these different properties is to apply multiple coats. It is common practice to coat primers as the first layer to provide good adhesion. After the primer is dried, a topcoat is then applied to afford more desirable surface properties. However, this approach consumes extra material, time, and effort. For applications that require both performance and fast turn-around, such as traffic coating, a simple one coat solution is strongly preferred. Another grand challenge in waterborne coating materials is to eliminate VOCs and create a “zero-VOC” paint. Such coatings will further benefit environmental and consumer health. However, removing all the VOCs will make it difficult for latex particles to form an integral coating film unless the glass transition temperature (Tg) for polymer binder is greatly reduced. This can be done by altering the polymer chemistry or adding non-evaporative coalescent molecules, however doing so will inevitably hurt coating hardness and many other properties. Therefore, technology that can guarantee film formation while providing a hard coating surface becomes the holy grail in coating research. Most ideas have been developed around two-component (2K) systems and crosslinking chemistry, which are usually much more costly and require more complicated chemistry and formulation.
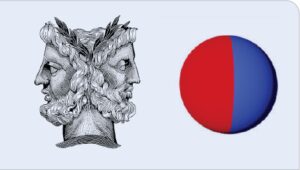
In this article, we report on an innovative coating additive, Janus particles, which can significantly modify the surface properties of a waterborne coating system while leaving the bulk of its properties mostly intact. As shown in Figure 1, Janus is the name of an ancient Roman god who has two faces. Janus particles are particles that possess two different chemical make-ups, often with contrasting properties, on each side of a single particle. For example, an amphiphilic Janus particle has one hydrophilic side and one hydrophobic side, which can also be viewed as a colloidal version of a small surfactant molecule. Although Janus particles have not yet been widely used in industry, they have been studied and developed in academia for more than 30 years.
FIGURE 1. Roman God Janus and Janus particles.
The Janus particle idea was first coined by P.G. de Gennes in his Nobel laureate lecture, where he raised the concept of soft matter.2 He listed four different types of soft matter: polymer, surfactant, liquid crystal, and the Janus particle. In de Gennes’ view, these materials are all “soft” because they are flexible and can form complex structures. The initial research progress on Janus particles was lacking due to their difficulty in synthesis. It is not straightforward to fabricate two surfaces or compositions of completely opposite properties on a single particle. Early methods involved masking part of the particles or using directional coatings to coat only half of the particles.3 These methods can produce Janus particles with well-defined morphology, although the yield is very limited. Even with a very small quantity, the studies have shown that Janus particles form unique self-assembly structures and adsorb strongly at the interface. These particles also demonstrated intriguing behaviors in the external fields.4-5 When one side of the Janus particles reacts with the surrounding environment and produces gas molecules, they become self-propelled active particles.6 However, the interesting discoveries in the academic world did not simply translate to successful commercialization and broad applications in industry. After all, it was difficult to scale up the Janus particle synthesis. It is also not clear how these particles can be utilized for real-world applications.
Continue reading in the January-February 2024 digital issue of CoatingsTech.