By Steffen Onclin, Huiguang Kou, Frank Pirrung,Rainer Diehl, Elena Martinez, and Clemens Auschra, BASF SE, Germany
and Andrew Recker and Anthony Moy, BASF Corporation, USA
Even though formulation additives comprise only a minor part of a coating formulation, they can have pronounced effects on the physical properties of the final coating. This is especially true for pigment dispersants, which can enter a coating formulation “under the radar” through, for example, pigment pastes. As dispersants are often polar polymers with a low glass transition temperature, the interface between the pigment and continuous phase forms a weak point, especially in coatings designed for high durability. This article addresses the development of an improved dispersant technology. The dispersants are functionalized such that they become part of the continuous phase of the coating, improving overall film integrity. Application examples, in both waterborne and solventborne formulations, demonstrate the effects of reactive technology on mechanical and barrier properties in comparison to conventional systems.
Introduction
Pigment dispersants form an essential component of a pigmented coating formulation. Their function is three-fold. First, dispersants help to wet the pigment surface by reducing the surface tension of the liquid formulation. This supports the penetration of the liquid into the interstitial volumes of the pigment powder to remove air and water from the pigment surface.1 Second, dispersants are designed to have an affinity for the pigment surface. During the grinding process, dispersants will adsorb onto the pigment particles and stabilize the particles, preventing re-aggregation and re-agglomeration. By choosing the right dispersant-pigment combination, the stabilization action allows for the preparation of stable pigment grinds with a particle size below 100 nm, approaching the primary particle size of transparent pigments. The third function of the dispersant is to provide compatibility between the dispersed pigment and the let-down phase of a coating formulation. This is especially critical when working with resin-free pigment pastes. The composition of the dispersant determines, to a large extent, the compatibility with the main resin system, which can have a marked effect on properties such as transparency, gloss, and color development.
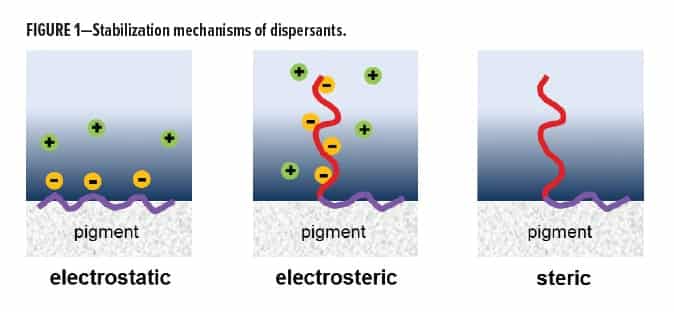 Pigment dispersant architectures typically consist of two parts. One part has a strong affinity for the pigment, while the other part stabilizes against re-aggregation. The stabilization part can be either ionic or steric in nature or a combination of both. This is schematically shown in Figure 1. While all three stabilization mechanisms are important in waterborne applications, dispersions in solventborne formulations usually rely on steric stabilization. Especially when transparency or high-jetness values are of importance, steric stabilization is the dominant stabilization mechanism, presumably because it can provide best compatibility with the main resin system.
Pigment dispersant architectures typically consist of two parts. One part has a strong affinity for the pigment, while the other part stabilizes against re-aggregation. The stabilization part can be either ionic or steric in nature or a combination of both. This is schematically shown in Figure 1. While all three stabilization mechanisms are important in waterborne applications, dispersions in solventborne formulations usually rely on steric stabilization. Especially when transparency or high-jetness values are of importance, steric stabilization is the dominant stabilization mechanism, presumably because it can provide best compatibility with the main resin system.
Steric stabilization is most effective when the stabilizing chains are sufficiently bulky, which means that high-molecular weight dispersants are preferred. A range of technologies is available to prepare high-molecular weight polymers, including polycondensation (e.g., polyurethanes) and ring-opening polymerization. Another well-known class of polymers is based on radical polymerization of (meth)acrylic- or vinyl monomers. These dispersants are often prepared by conventional free-radical polymerization. However, this approach is limited to random copolymer structures and does not allow further enhancement of the stabilizing mechanism through more complex polymer architectures. More recently, controlled free-radical polymerization has become available at the industrial scale and allows for the synthesis of new polymer architectures, like block structures, which are not accessible by conventional radical polymerization techniques.2-4
Effect of Dispersants on Coatings Properties
Dispersants are designed to have a strong affinity for pigment surfaces and to provide stabilization against re-aggregation of the dispersed pigment particles. The development of new dispersants most often focuses on improving traditional effects, such as coloristics or grinding efficiency. Grinding resins, on the other hand, are also widely used to disperse pigments. The demarcation between dispersants and grinding resins is not sharp, but in general, grinding resins are developed from main resin technologies, focusing more on coatings properties than on pigment dispersion. Figure 2 shows the rheological behavior of a solventborne e-phthalocyanine blue pigment paste. The paste prepared with grinding resin as single dispersing active shows relatively poor viscosity control in the low shear region. Exchanging part of the grinding resin with a dedicated commercial dispersant [internal benchmark (BM) 1] improves the rheological behavior. After letdown of the pigment pastes in a medium-solids (MS) OEM basecoat, the transparency of the applied coatings was measured (Figure 2). An improvement in transparency was observed by exchanging part of the grinding resin with internal BM 1.
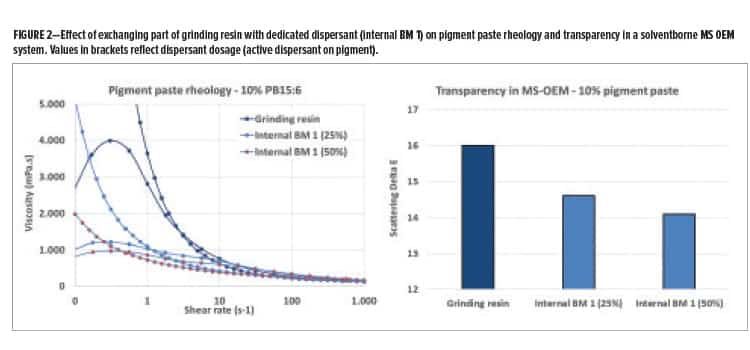
As anticipated, the addition of internal BM 1 provides improved pigment paste rheology and transparency of the final coating, which are focus areas during dispersant development. Many dispersants, including high-molecular weight dispersants, are low Tg polymers that typically do not carry any functionality and, therefore, will not become part of the continuous resin phase. The dispersed pigment particle thus forms an inhomogeneity in the continuous phase of the coatings, which could lead to reduced film integrity. The dispersant, located at the interface between the pigment particle and the continuous phase, could play a decisive role regarding barrier properties of the final coating. Figure 3 shows the effect of pigment paste addition on the hardness and MEK-rub resistance of an MS-OEM basecoat. The pigment paste containing grinding resin as the single dispersing active has no noticeable influence on coating hardness and provides good MEK-rub resistance, which is indicative of a highly crosslinked system. The addition of the pigment pastes that contain internal BM 1, which does not react with the main resins, shows a softening effect especially at a higher dosage level. Additionally, the basecoat swells quickly during the MEK-rub test leading to poor resistance results.
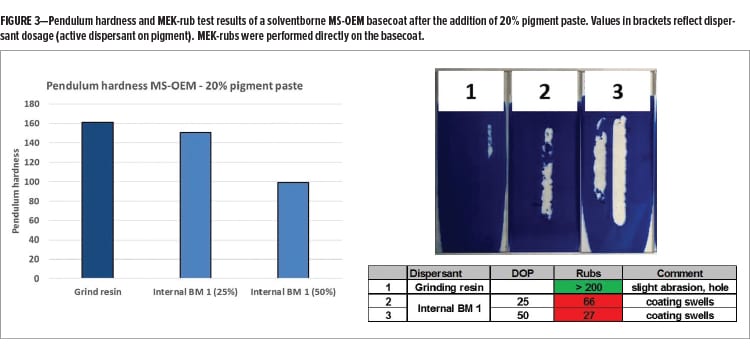
These results show that the addition of non-functional dispersant can result in a plasticizing effect of the final coating, which can lead to reduced barrier properties. In this article, we explore the possibility of developing a dispersant that can react with the main resin matrix, reducing negative effects on coatings properties, but without compromising the dispersing properties.
Dispersant with New Anchoring Chemistry
One key requirement for a dispersant to provide optimal dispersing properties is to have the right pigment affinity groups. Strong pigment affinity often leads to improved dispersing efficiency, better rheology control, and improved coloristics.5,6 Here, our goal was to develop a new solventborne dispersant for carbon blacks and transparent organic pigments following the automotive trends towards deeper blacks and highly chromatic, transparent colors. In particular, high-color carbon blacks, with a primary particle size far below 50 nm, are difficult to stabilize.
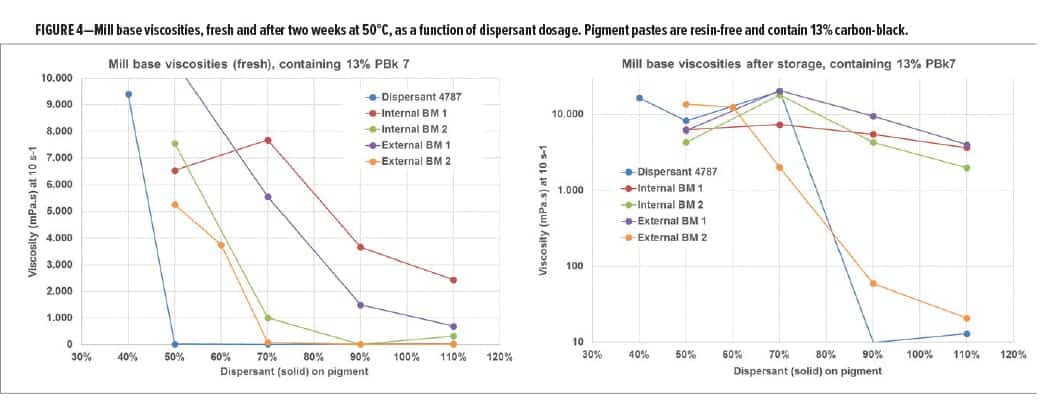 Detailed analytical and application studies resulted in the development of a of new pigment affinity chemistry that is used in a new dispersant, referred to as Dispersant 4787. This new dispersant was evaluated in a comparative study with two internal and two external benchmarks. Figure 4 shows the viscosity of resin-free pigment concentrates that contain 13% carbon black. The viscosity at a 10 s-1 is plotted against the dispersant dosage, showing dispersant efficiency in viscosity suppression. A strong spreading of results was found. Dispersant 4787 already reached low viscosity at a dosage of 50% active dispersant on pigment (DOP) while the commercial benchmarks needed either a higher dosage or did not even reach low viscosity at the highest dosage. The data after storage show the main challenge when working with carbon-black pigment pastes: it is difficult to stabilize the particles over longer time periods. Only Dispersant 4787 and one competitor could provide low-viscous pigment pastes after storage at 50°C.
Detailed analytical and application studies resulted in the development of a of new pigment affinity chemistry that is used in a new dispersant, referred to as Dispersant 4787. This new dispersant was evaluated in a comparative study with two internal and two external benchmarks. Figure 4 shows the viscosity of resin-free pigment concentrates that contain 13% carbon black. The viscosity at a 10 s-1 is plotted against the dispersant dosage, showing dispersant efficiency in viscosity suppression. A strong spreading of results was found. Dispersant 4787 already reached low viscosity at a dosage of 50% active dispersant on pigment (DOP) while the commercial benchmarks needed either a higher dosage or did not even reach low viscosity at the highest dosage. The data after storage show the main challenge when working with carbon-black pigment pastes: it is difficult to stabilize the particles over longer time periods. Only Dispersant 4787 and one competitor could provide low-viscous pigment pastes after storage at 50°C.
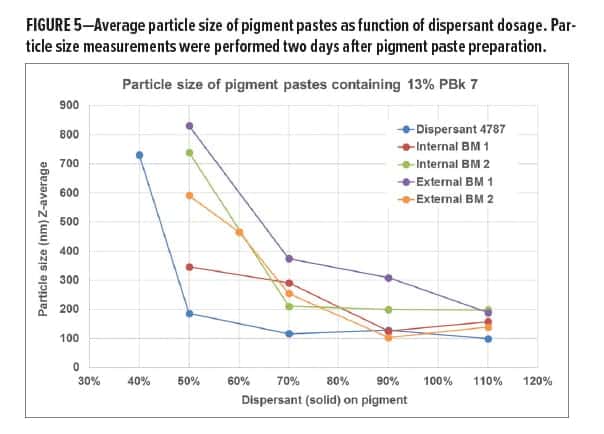
Particle size measurements revealed a similar trend. Figure 5 shows that Dispersant 4787 reaches small particle size at a lower dosage when compared to the commercial benchmarks. Although fresh rheology data and particle size measurements correlate well for Dispersant 4787, this is not the case for every dispersant. Internal BM 1, for example, gave relatively high-viscous pigment pastes at all dosage levels but provided small particle size at 90% DOP. This indicates that the polyester stabilizer group chemistry, which is used in Dispersant 4787, is particularly effective in viscosity reduction when compared to acrylic-based internal BM 1.
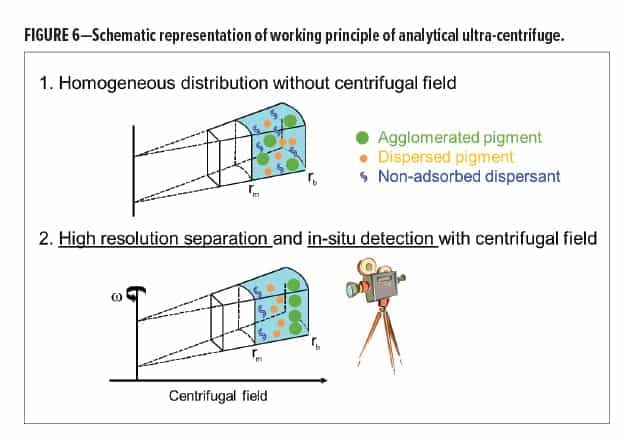
To gain a deeper understanding of the interactions between dispersant and pigment, adsorption studies were performed.2,7-9 An analytical ultra-centrifuge (AUC) was used for adsorption quantification. This technique relies on the high-resolution separation and in-situ detection of the species that are present in the pigment pastes in a centrifugal field. Without a centrifugal field, all components in a pigment paste are homogeneously distributed, making it impossible to obtain information on the individual components. However, when a centrifugal field is applied, time-resolved concentration profiles provide information on the different constituents of the pigment paste. This is schematically shown in Figure 6.
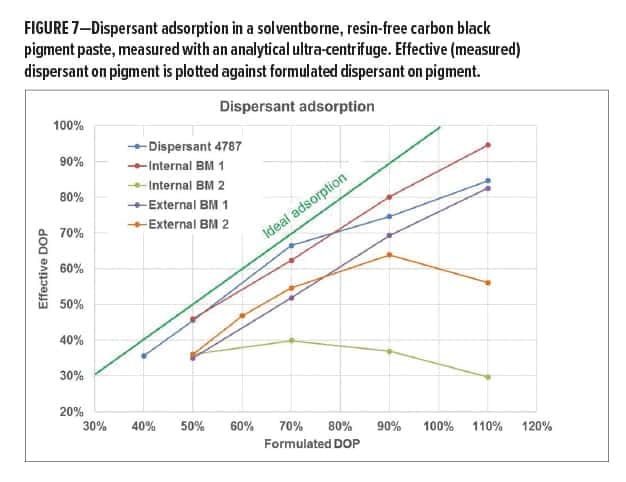
Such AUC measurements provide information on the amount of dispersant that is adsorbed onto the pigment surface versus unbound material. With this information, dispersant adsorption diagrams, as shown in Figure 7, can be prepared. The diagram plots the effective (= measured by AUC) dispersant on pigment amount against the formulated dispersant on pigment amount. If all dispersant that was added to the pigment paste (= formulated DOP) would be adsorbed onto the pigment surface, the result would be the ideal adsorption line shown in green. This would mean that no unbound material would be present in the pigment paste. In reality, the different tested dispersants show a large spreading in adsorption strength. Internal BM 1 and Dispersant 4787 show close to ideal adsorption, especially at lower DOP levels, indicative of strong interactions between the pigment affinity groups of the dispersant and the pigment surface. More conventional adsorption behavior can be observed for external BM 2. After an increase in adsorption at lower dispersant dosage levels, the adsorption curve enters a saturation level where additionally offered dispersant does not result in additional adsorption.
Next, the pigment pastes were mixed with different letdown systems and applied to measure jetness and undertone. It was evaluated to what extent the properties of the pigment pastes are transferred into the final coating. Figure 8 shows jetness and undertone values in an MS-OEM basecoat. Consistent with rheology and particle size data, Dispersant 4787 already shows high-jetness values and a good bluish undertone at 50% dosage level, which is not the case for the tested commercial benchmarks. For all tested dispersants, the optimal dosage is 90% or 110% active dispersant on pigment reflecting the large pigment surface that needs to be stabilized with such a small-sized particle.
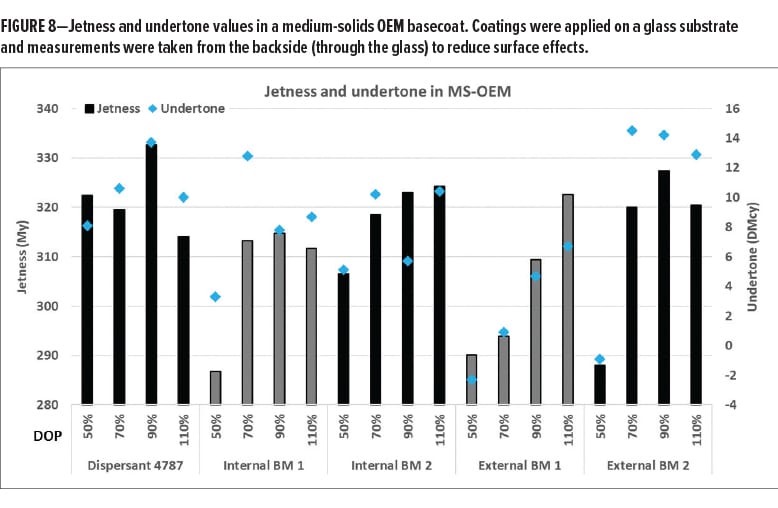
Figure 9 shows the jetness and undertone results in a two-component polyurethane (2KPU) topcoat. The effect of dispersant dosage on jetness and undertone is clearly visible. Similar to the results in an OEM basecoat, Dispersant 4787 provides already good results at low dispersant dosage and highest jetness levels at optimal dispersant dosage.
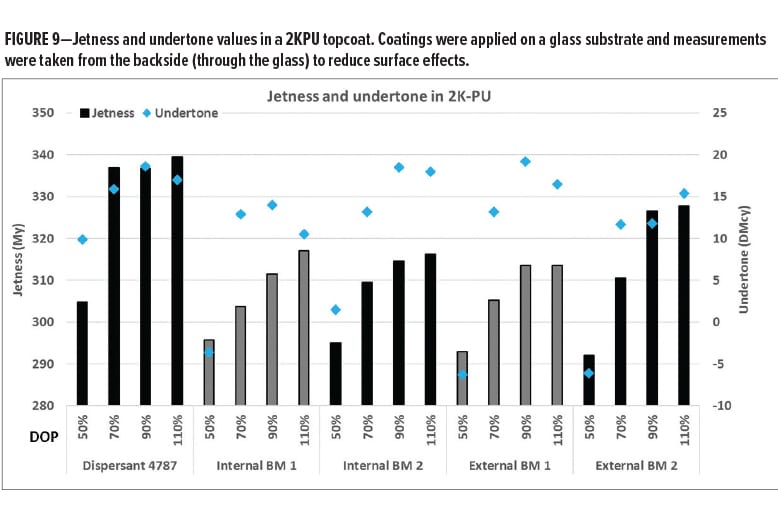
In summary, the anchoring chemistry optimization resulted in the development of a new dispersant with improved efficiency over existing internal and external benchmarks. We believe that the improved adsorption of the new anchoring chemistry, as reflected by AUC studies, is key to achieve this improved efficiency. Ongoing studies with transparent organic pigments show that the new anchoring chemistry has a strong affinity to a wide range of different pigment surfaces.
Reducing Negative Impact of Dispersants on Coating Properties
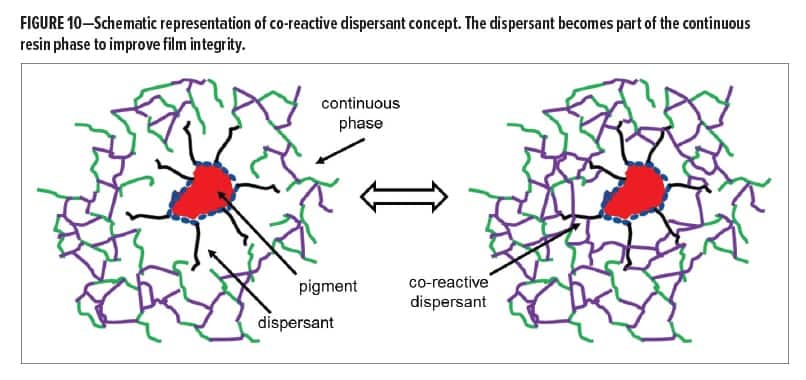
As described in the section on “Effect of Dispersants on Coatings Properties,” dispersants are typically designed to have a positive effect on rheological properties of pigment pastes and coloristic properties in the applied coating. They can, however, negatively affect the physical properties of the final coating. Figure 10 shows a schematic representation of a dispersed pigment particle in a coating. Dispersants often do not carry functional groups to become part of the continuous resin phase, which means that the pigment particle with dispersant at the interface forms a foreign body. Especially at higher pigment loading, or at high dispersant dosage, this can have a measurable effect on the physical properties of the coating as was shown in Figure 3. This part of the article describes our efforts to functionalize dispersants with reactive groups to become part of the continuous resin phase and evaluate whether negative effects on physical properties can be reduced.
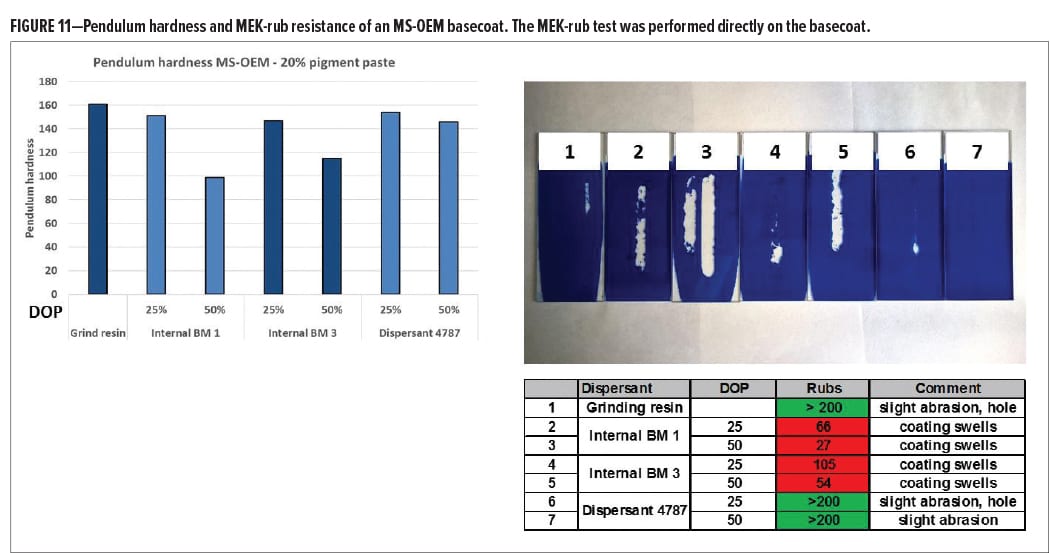
To evaluate the effect of dispersant crosslinking, ε-phthalocyanine blue pigment pastes were prepared. One pigment paste contained grinding resin as a single dispersing active. The grinding resin contains functional groups and will form a network with the main resins. In the other pigment pastes, part of the grinding resin was substituted by dispersant. Internal BM 1 is an acrylic dispersant with no functional groups. Internal BM 3 is a modified polyester dispersant with no functional groups. Dispersant 4787 is, like internal BM 3, based on a modified polyester but contains hydroxyl functional groups. Figure 11 shows the pendulum hardness and MEK-rubs results after application in an MS-OEM basecoat. The dispersants with no functionality cause a softening effect of the coating as measured by lower pendulum hardness, especially at higher dispersant dosage. The pigment paste that contains OH-functional Dispersant 4787, however, hardly affects the coating hardness. MEK-rub resistance was tested directly on the basecoat. The results are shown in the picture and table in Figure 11. The MEK-rub test was performed directly on the basecoat. The coating containing the pigment with only grinding resin gave good MEK-rub resistance; after 200 double-rubs, only a slight abrasion was observed. When the dispersants without functionality were used, a dramatic impact on solvent resistance was observed. Immediately after starting the test cycle, the coating started to swell, resulting in poor MEK-rub resistance. This effect was not visible when Dispersant 4787 was used and good MEK-rub resistance was found even at high dispersant dosage. These results indicate that the functional groups in Dispersant 4787 indeed become part of the main resin matrix maintaining a high crosslink density and providing enough barrier properties to prevent solvents from penetrating the coating.
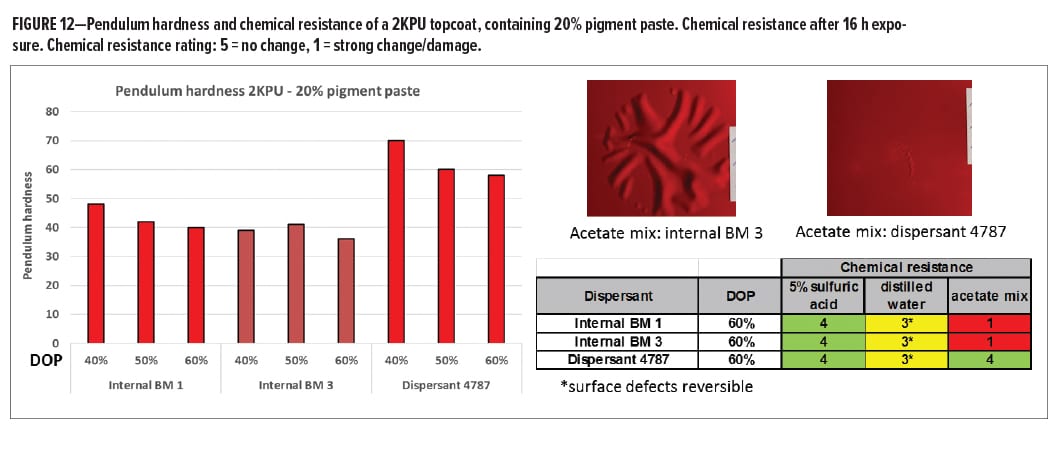
Similar experiments were performed on a 2KPU topcoat. First, resin-free pigment pastes with PR 254 were prepared at three dispersant levels. Two dispersants that carry no functionality were compared with Dispersant 4787. These pigment pastes were mixed with the 2KPU letdown and applied. A pigment paste loading of 20% was chosen. The first indication of the reactivity of Dispersant 4787 was provided by pendulum hardness measurements. Figure 12 shows the hardness four days after application and curing. A significantly higher hardness was observed for the coating containing the paste based on Dispersant 4787 indicating that this dispersant contributes significantly to crosslink density. The amount of dispersant present in the final coating ranges from 3.6% on total polymer when the paste with 40% DOP is used, to 5.3% for the paste with highest dispersant dosage. This suggests that small amounts of a functional additive can cause significant effects in the final coatings system. Chemical resistance of the coatings was examined by placing a cotton batting soaked with the chemical of choice on the surface of the coating for 16 h. After removal of the cotton batting and cleaning of the surface, the damage to the coating was evaluated. The results are shown in Figure 12. No difference in resistance was observed for aqueous chemicals, which could be explained by the hydrophobic character of the resins used. However, strong differences were observed with an organic solvent mix. When a dispersant was used that is not reactive, the coating was swollen and detached from the substrate. Using Dispersant 4787, hardly any change in the surface was found, indicating good barrier properties against organic solvents.
The results described above suggest that the functionality on Dispersant 4787 is accessible for reaction with the main resins, the dispersant, therefore, becomes part of the continuous phase. Even when dispersants form only a minor part of a coating formulation, significant effects in hardness and chemical resistance could be observed reducing negative impact in comparison to non-functional counterparts.
Towards Waterborne Reactive Dispersants
The conversion to waterborne technologies continues to be one of the major trends in the coatings industry. Nowadays, the major conversion is taking place in the Asia Pacific region especially focusing on industrial applications. Achieving durability properties that are comparable to solventborne technologies remains a challenge where additives usually play a negative role. Waterborne additives are typically polar, low Tg materials that can have a marked softening effect in the final coating, migrate, and increase water sensitivity. To reduce such negative effects, dispersants were synthesized with hydroxyl functional groups in the stabilizer part of the polymer. The hydroxyl groups can react with polyurethane and melamine crosslinking systems during curing. It is hypothesized that when the dispersant is immobilized and becomes part of the main resin system, negative effects on durability properties could be reduced.
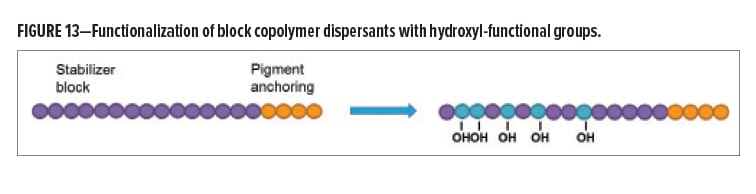
To prove this concept, OH-functional polymers based on controlled-free radical polymerization were prepared. With this technology, block copolymer dispersants can be prepared where each block has a distinct function. For this study, the pigment anchoring block of the dispersants was not modified, and the functionality was built into the stabilizer block of the polymer. This part of the polymer is thought to move away from the pigment surface to provide steric stabilization. It has good compatibility with a range of aqueous resin systems, which increases the likelihood of a reaction. The prepared dispersants are based on a commercially available block copolymer that carries no functionality. The general structure is schematically shown in Figure 13.
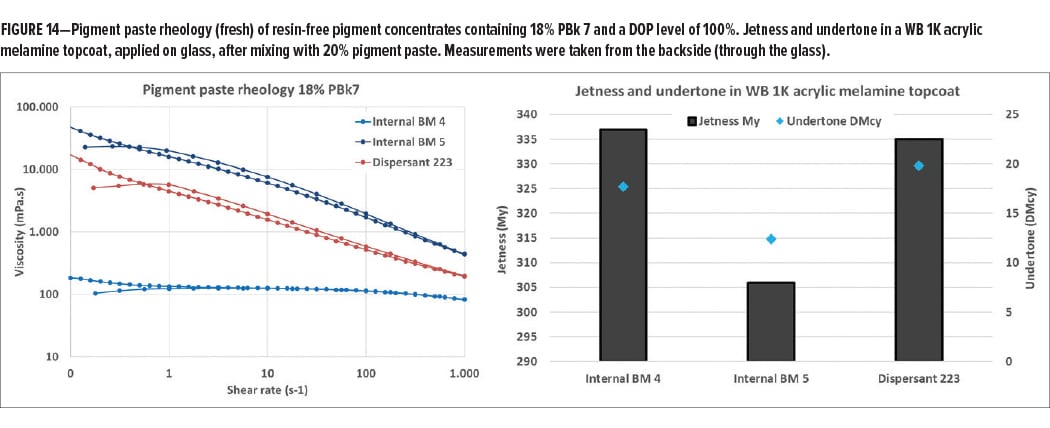
After synthesis, the first step was to test the dispersing properties of the new dispersants. To this end, a commercial product (internal BM 4) was compared to two experimental grades that are chemically very close. Internal BM 5 is an experimental dispersant that carries no functional groups and Dispersant 223 is based on the same chemistry, but with hydroxyl functionalities. Figure 14 shows the rheological behavior of resin-free pigment pastes containing 18% carbon black at an active dispersant dosage of 100%. The experimental grades are not able to provide the low-viscous, Newtonian behavior of the commercial counterpart, but still give an acceptable rheology. The second part of Figure 14 shows jetness values after letdown in a waterborne 1K acrylic melamine topcoat. In this case, experimental Dispersant 223 provides excellent jetness values and bluish undertone, which means that the OH-functionalization kept the dispersing properties intact.
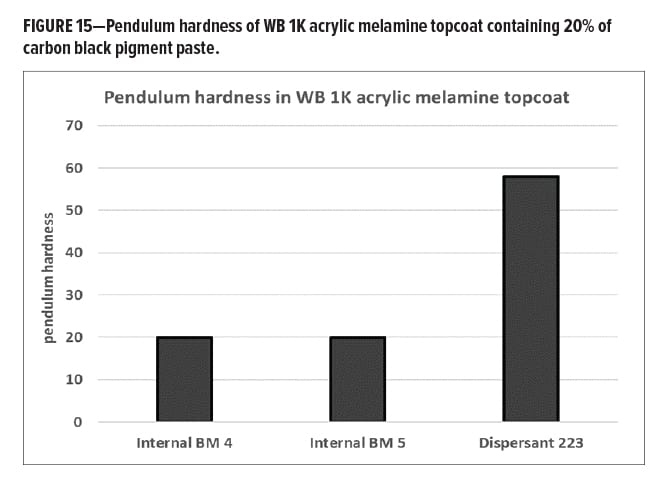
To assess if the OH functionality on Dispersant 223 has any measurable effect on coatings properties, pendulum hardness was measured. Figure 15 shows that addition of pigment paste containing non-functional dispersant results in very soft coatings exemplified by the addition of pastes containing internal BMs 4 and 5. However, the pigment paste containing OH-functional Dispersant 223 provides a much higher pendulum hardness value. This is a strong indication that the OH-functional dispersant becomes part of the resin matrix and, in this way, maintains high crosslink density. The dispersant level in the final coating was high, ca. 9% on total polymer, which could be an explanation for the magnitude of the observed differences.
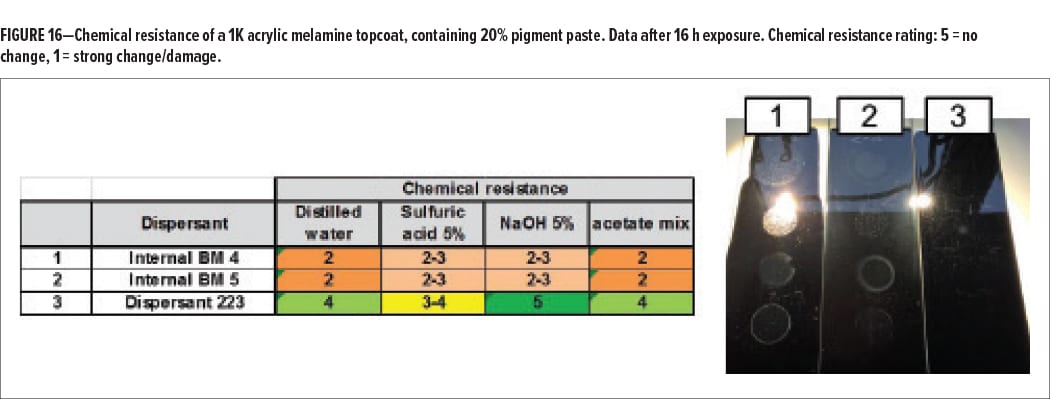
In a second step, the chemical resistance properties of the tinted coatings were examined. The coatings were exposed to three aqueous and one organic liquid by placing a soaked cotton batting on the surface for 16 h. After exposure and rinsing, the surface of the coatings was evaluated. Figure 16 shows the results in table form and in a picture. A significant difference in barrier properties was found. The surface of the coatings that contained non-functional dispersant showed extensive, irreversible damage with all tested liquids, which indicates penetration into the coating. The coating surface formulated with Dispersant 223, on the other hand, showed much less damage. Significant surface modification was found only with 5% sulfuric acid.
These first results indicate that the use of OH-functional Dispersant 223, like Dispersant 4787 for solventborne applications, reduces negative effects on final coating applications by becoming part of the continuous phase. Extensive softening of the coating is prevented and improved barrier properties against aqueous and organic liquids is obtained.
Conclusions and Outlook
Pigment dispersants form an essential part of a pigmented coating formulation. Their use normally has a positive influence on properties such as grinding efficiency, color development, and transparency. Physical properties of the coating are, however, often negatively impacted by these additives. Here, we reported on our efforts to develop a new solventborne dispersant, Dispersant 4787, which provides the possibility to crosslink with the main resin matrix. Using this concept, it was shown that a dispersant can, in fact, achieve excellent dispersing efficiency while also significantly reducing the usual negative impact on coating properties, such as solvent resistance and hardness. First trials with a co-reactive waterborne block-copolymer indicated that similar effects can be achieved in waterborne formulations.
We believe that the use of co-reactive dispersant technology can assist formulators to overcome formulation or application constraints either directly, as higher amounts of dispersant can be tolerated, or indirectly, by being able to use a wider range of raw materials that lead to critical coating properties in combination with non-reactive dispersants.
References
- Bieleman, J., Additives for Coatings, Wiley-VCH, Weinheim, 1998.
- van den Haak, H.J.W., J. Coat. Technol., Vol. 69 (873), pp. 139-144 (1997).
- Pirrung, F.O.H., Quednau, P.H., and Auschra, C., Chimia, Vol. 56 (5), pp. 170-176 (2002).
- Auschra, C., Eckstein, E., Muhlebach, A., Zink, M.O., and Rime, F., Prog. Org. Coat., Vol. 45 (2-3), pp. 83-93 (2002).
- Auschra, C., Eckstein, E., Knischka, R., Pirrung, F., and Harbers, P., Eur. Coat. J., pp. 26-35 (2004).
- Auschra, C., Eckstein, E., Knischka, R., Pirrung, F., Harbers, P., Surf. Coat. Intern. Part A, Vol. 8, pp. 377-383 (2006).
- Legrand, P., Eur. Coat. J., Vol. 3, p. 90 (2005).
- Huisman, H.F., Pigmans, H.J.M., Roegies, A.J.P., and Sprangers, W.J.J.M., Prog. Org. Coat., Vol. 13, p. 377 (1986).
- Řeháček, K., Farbe und Lack, Vol. 76, p. 656 (1970).
Presented at the American Coatings CONFERENCE, April 9–11, in Indianapolis, IN.
CoatingsTech | Vol. 15, No. 7 | July 2018
