By Ingrid K. Meier, Fadia Namous, and K. Michael Peck, Evonik Corporation and Roger Reinartz, Evonik Resource Efficiency GmbH
Siloxane-based additives are critical tools in coating applications because their structures can be varied to provide a broad range of performance benefits in many types of formulations and chemistries. Surfactants and defoamers are some of the more commonly recognized additive classes, but many other functionalities can be derived from siloxane chemistries, particularly attributes related to surface control such as flow and leveling, slip, scratch resistance, and haptic properties. This extensive range of performance attributes is achievable due to the broad flexibility inherent in siloxane chemistry, allowing a fine-tuned balance of compatibility, incompatibility, and surface activity.
As with many additive types, a broad range of functionalities creates many options for improvement and innovation but also presents challenges in finding the right additive and optimizing to achieve the desired performance. This article will attempt to clarify the general structure-
property relationships that drive the performance attributes of siloxane additives and detail the continuum that exists between wetting, leveling, defoaming, and slip within this chemistry class. Surface control properties and testing will be reviewed and related to recent evaluation work conducted in developing novel siloxane surface control additives.
Introduction
Siloxane Chemistry
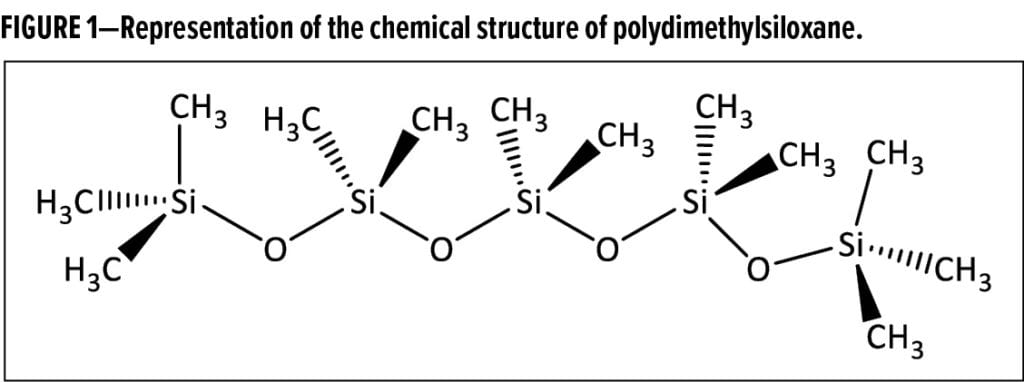
Siloxanes are molecules that contain Si–O–Si linkages, and their structures can be tailored to create materials with a broad spectrum of properties. While siloxanes can be derivatized with many different organo-functional groups to create a variety of small molecules and polymers, the siloxane backbone itself contributes some of the most unique attributes. The structure of underivatized polydimethylsiloxane (Figure 1) illustrates how this backbone, comprised of repeating –Si(CH3)2–O– units, results in a molecule that is both highly methylated and extremely flexible. It is this feature of siloxanes that enables their high level of surface activity because the surface energy of a “methyl-
saturated” surface is ~20 mN/m.1-3
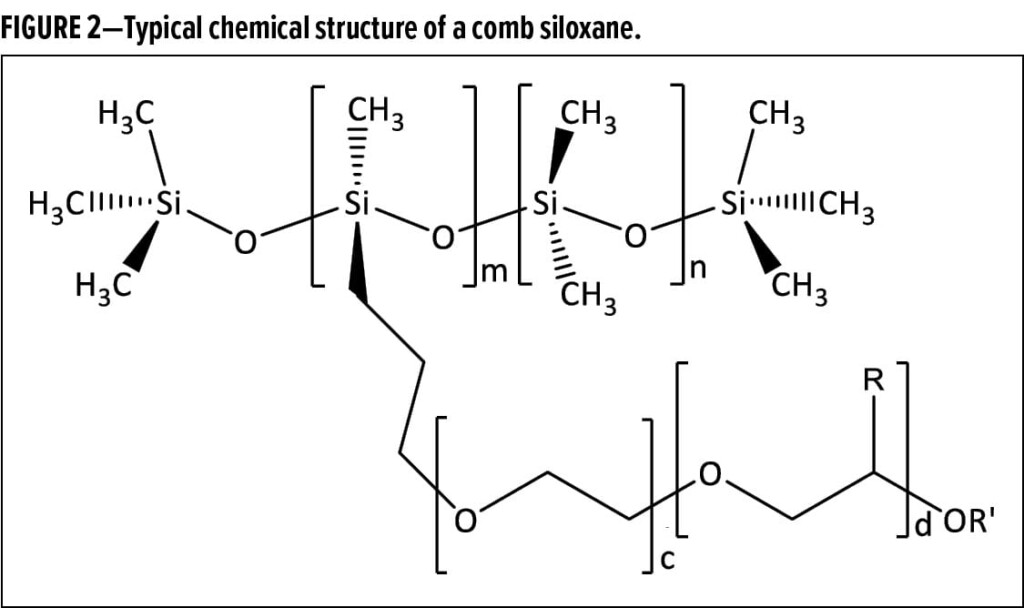
Siloxane-based wetting agents are generally smaller molecules with relatively short siloxane backbones. As shown in Figure 2, they are typically either trisiloxanes (n = 0, m = 1) or have comb structures with very few –Si(CH3)2–O– units (n) and only a very few organo-modifications (m); generally, n + m is less than 5. Hydrophilicity can be tuned by varying the nature and length of the pendant organic groups and by modifying the ratio of hydrophilic and hydrophobic moieties present in the molecule. In aqueous systems, these amphiphilic siloxanes are driven to the air–water interface and can lower surface tension within a time frame consistent with their molecular mobility. Molecules with longer siloxane backbones can also be rendered oleophobic and may also be driven to the air–liquid interface in nonaqueous, organic-based systems. Siloxane-based wetting agents are most often used to lower the surface tension of aqueous and solventborne, as well as high-solids and 100% solids, formulations to improve substrate wetting. Occasionally, these wetting agents are also used to compatibilize insoluble oils—particularly silicone contaminants—to prevent problems such as dewetting and defects.
Siloxane defoamers can also be created using comb siloxane structures like that shown in Figure 2; however, siloxane defoamer molecules are higher in molecular weight than siloxane wetting agents, and they usually have longer
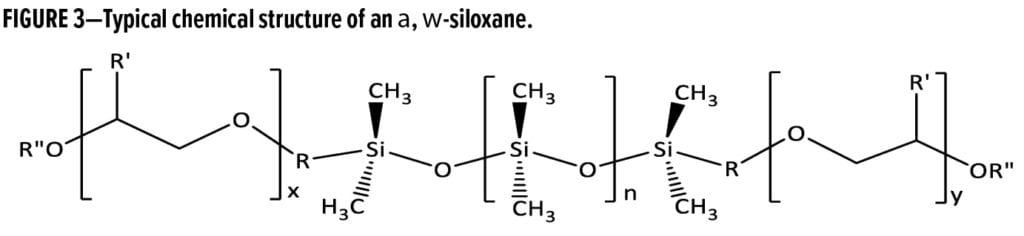
–Si(CH3)2–O– segments (larger n) as well as more pendant groups (m) that are more hydrophobic in nature (i.e., c = 0 or d > c). A wide variety of molecular structures can be synthesized via formation of Si–O–C or Si–C linkages. Linear siloxanes like that shown in Figure 3 (also known as a, w- or bolaform structures) can be used to create defoamers and deaerators that range in performance attributes as the length of the siloxane backbone and lengths and chemical natures of organo-modifications are varied. In general, defoamers are designed to be incompatible in the surrounding media because this enables them to be driven to the lamellae of foam bubbles where they can spread and destabilize them, causing bubble rupture.
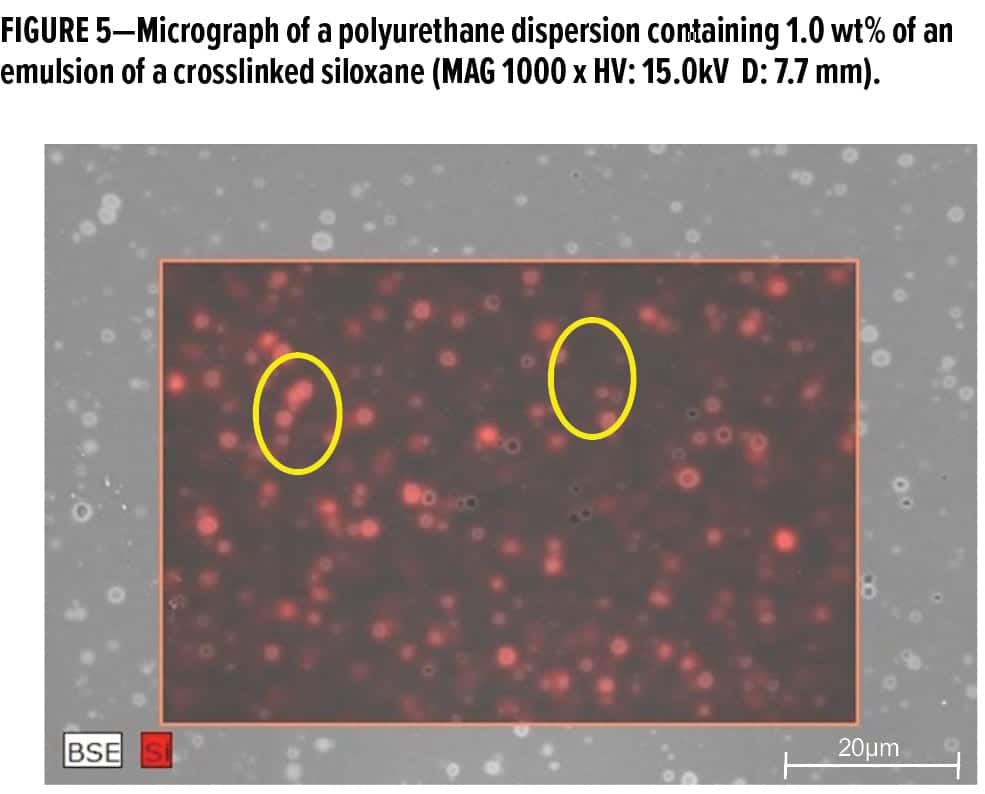
As one increases the length of the siloxane block within a molecule, the interfacial activity of the molecule also increases. Therefore, polyether-modified siloxanes that can improve flow and leveling, increase surface slip, and impact other surface properties are also possible. These surface control additives are generally of moderate molecular weight (between 1,000–20,000 g/mol), and compatibility within the intended liquid matrix is achieved through a diversity of organo-modifications. Thus, surface control additives that function well in waterborne, solvent-based, and 100% active (solvent-free) systems now exist. Three examples of organo-modified siloxane-based surface control additive structures are shown in Figure 4. Additionally, it is possible to crosslink and emulsify or disperse siloxanes to create emulsions or dispersions of even higher molecular weight and more hydrophobic moieties. Unlike the 100% active liquid siloxanes that exist as nanometer-scale polymers, these siloxane emulsions or dispersions exist as micrometer-sized droplets or particles, as shown in Figure 5.
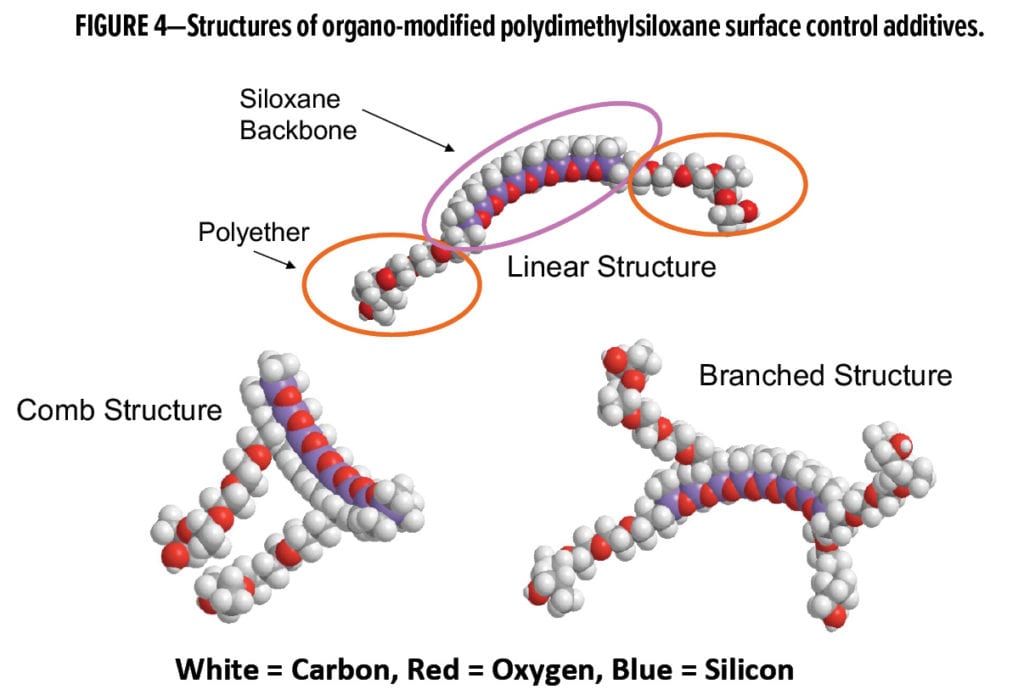
Siloxane-based Surface Control Additives in Coating Applications
Due to their unique interfacial activity, siloxane-based surface control additives have become invaluable tools in the coating formulator’s tool box. Many surface control additives are capable of mitigating surface energy gradients that can exist within a liquid film immediately after application; thus, they can improve flow, prevent retraction, minimize cratering, enable better surface leveling, and ensure a flawless surface appearance. As their surface activity increases, surface control additives can also render special properties to the surface of the coating. For example, depending on their chemical structures, certain surface control additives can impact surface slip, affect haptic properties (surface “feel”), impart scratch resistance, act as antiblocking agents, and even create a release effect on the surface of the cured coating.
The property of “slip” is characteristic of a smooth sliding motion across the coating that results from a reduced coefficient of friction. It is quantified as the force needed to slide a mass across the coating surface. The material properties of both the substrate and the object to be moved are reflected in the static and dynamic coefficients of friction, and the chemical composition of the coating and the interactions arising from it, as well as surface roughness, all contribute. Surface control additives with large polydimethylsiloxane segments and a high degree of surface activity can ensure particularly slippery surfaces. Additionally, coatings with a high slip often feel smooth and silky.4
Coatings may resist scratching when the scraping object slips off the surface rather than penetrating the coating film; however, force-dependent scratch resistance of a coating can only be significantly improved if the surface control additive used contains functional groups that do not interact strongly with one another.4,5 Organo-modified siloxanes, with a high percentage of polydimethylsiloxane domains, exhibit particularly weak interactions, both with each other and with other materials. This can make them ideal for this purpose. Moreover, during the drying process, organo-modified siloxanes continually migrate to the air–liquid interface, producing a lubricating film that significantly reduces the coefficient of friction of the coating. When a cured coating surface has a strong polydimethylsiloxane character due to the use of a surface control additive it is also more likely to resist blocking—adhesion of the dried coating to another freshly coated surface or other substrates. Liles has described the vast differences in, as well as the unique features of, silicone chemistry in two review articles; the reader is encouraged to consult these for additional background information on this fascinating topic. 6,7
The Need for Recoatability: The Balancing Act
While it is possible to achieve a variety of desired benefits by using siloxane-
based surface control additives that have large polydimethylsiloxane segments, the need for most coatings to be able to be eventually recoated remains. The downside to employing these incredibly hydrophobic and oleophobic surface control additives is the tendency for the resultant coating to have a very low surface energy and to be very difficult—if not impossible–to recoat. Even if a second coating layer can be applied to such a surface, lack of adhesion and the tendency for the second coating layer to show craters are true problems.6,7
To address these seemingly conflicting requirements, a series of new organo-modified siloxane-based surface control additives has been developed. This article will describe both the chemical attributes and the achievable benefits of these novel surface control additives.
Results and Discussion
Experimental
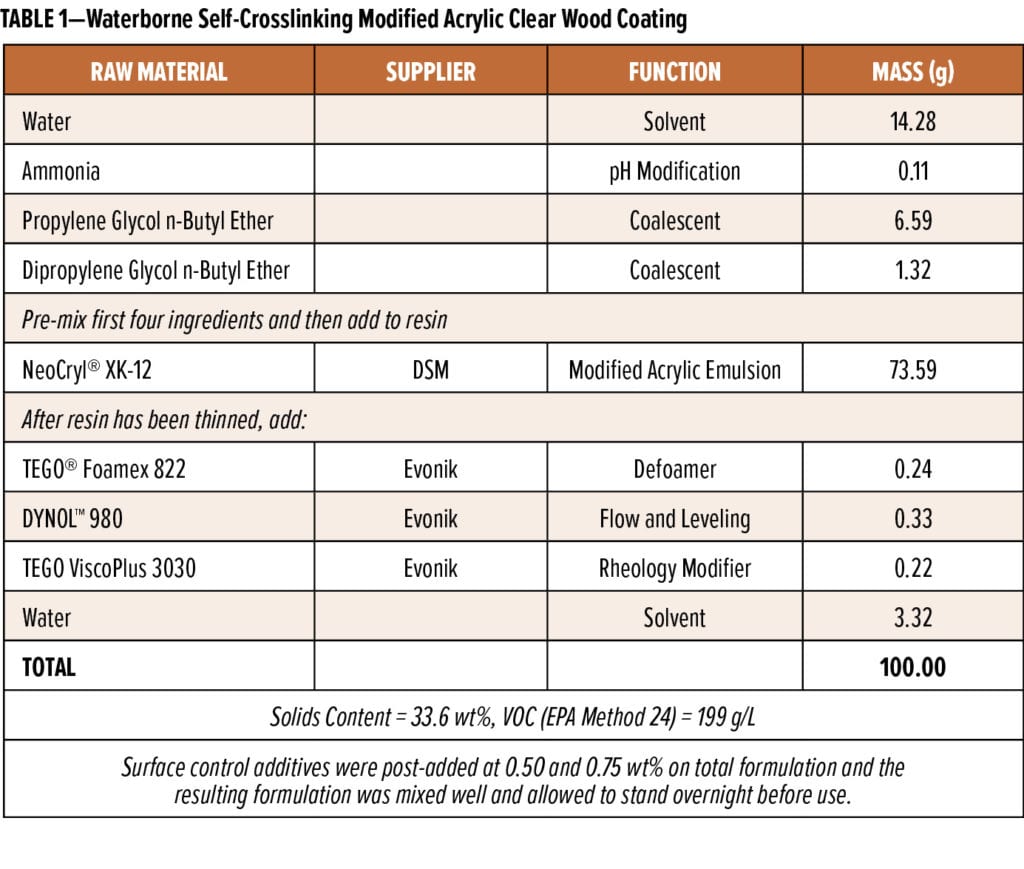
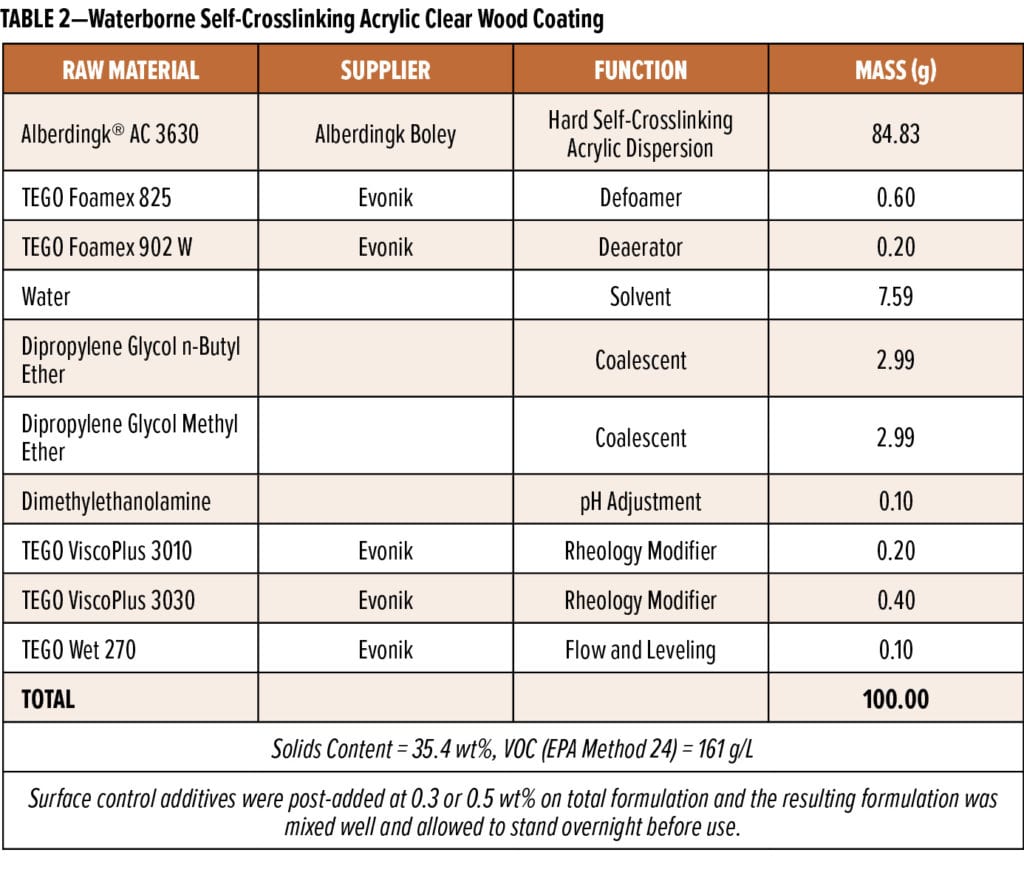
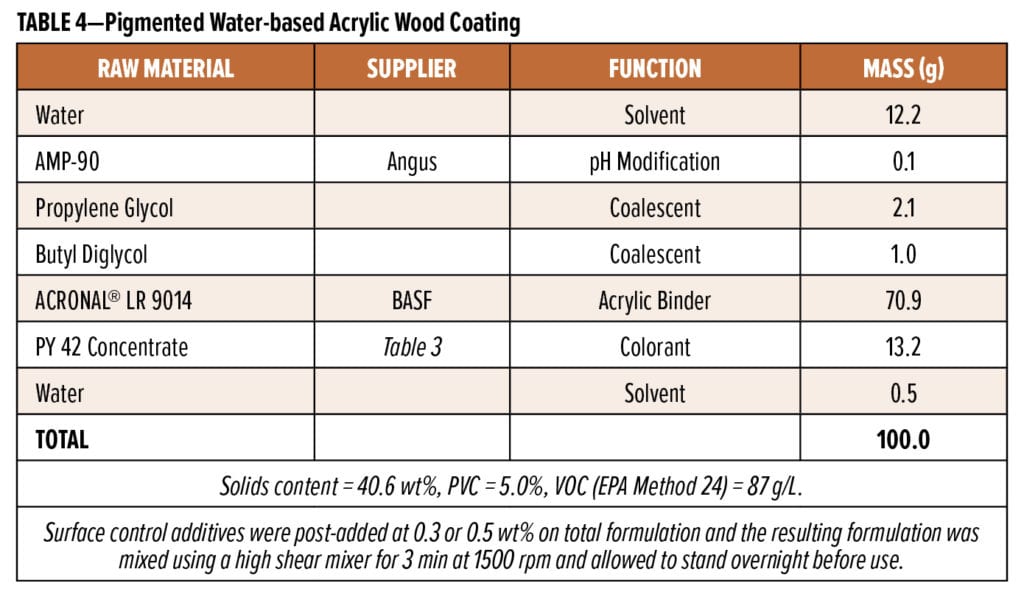
Three different waterborne self-crosslinking acrylic wood coating formulations were prepared using the formulations presented in Tables 1 through 4. Siloxane-based surface control additives were commercial products sold by Evonik Corporation under the TEGO® Glide brand, and they were used as received. The 35% active wax emulsion (AQUACER® 539) used as a control surface control additive in the formulation in Table 1 was a commercial product supplied by BYK USA. The water-based oil-modified polyurethane clear semi-gloss (MINWAX, Product code: 63020/71032) was purchased from a local retail store.
The coating formulations in Tables 1 and 2 were applied to plain black, sealed Leneta charts that measured 8 5/8 x 11 1/4 in.
(219 x 286 mm) using a #28 wire wound rod. After seven days of drying at ambient temperature and humidity, the coatings were inspected and evaluated for visual defects and ranked on a scale of 1 to 10 with 10 being perfect and 1 having many defects (Figure 6). Defects observed included pinholes, craters, de-wetting, orange peel, and smearing. Gloss measurements at 20°, 60°, and 85° were taken on the coated black Leneta charts using a Micro-TRI-gloss (Cat. No. 4446 Ser. No. 1083656) from BYK Additives & Instruments.

Relative Qualitative Slip was assessed by taping a Leneta chart coated (once) with the formulation containing no surface control additive and a second Leneta chart coated (once) with the comparative coating onto a single glass plate. A clean coin was placed on each chart and the glass plate was lifted relatively slowly until one of the coins slid off. This test was repeated several times to help assess whether the coated surface was truly more slippery than the blank. The coins were switched, and testing was repeated. The blank (no additive) coating was assigned a “0” and scores of “+” and “++” were given for increasing degrees of slipperiness while a “–” rating was assigned to coatings that were less slippery than the blank. Similar ratings were used for Relative Qualitative Smooth Feel. In these evaluations, the technician compared the feel of each coating and rendered a judgment based on her subjective opinion.
Red oak panels were lightly sanded using 220-grit fine sandpaper and they were brushed off with a clean high quality synthetic bristle brush before each application. Three panels were used for the blank formulation: two in the beginning and one at the end. The remaining panels were coated with the formulations containing 0.5 wt% of the surface control additives. The order of the coating process was randomized to reduce the effect of the operator. The brush was dipped into the coating sample and the entire panel was coated for the first coat. After two hours, the panel was lightly sanded, and 1 mL of the coating was pipetted to the panel and evenly distributed using the designated brush in a back and forth motion. This process was repeated after another two hours but 2 mL were added in the final coat. The brush was placed in aluminum foil and tightly sealed between each coat to avoid drying. The panels were left to dry for about five days before scratch and mar testing.
Scratch resistance was evaluated using an internally developed method (Figure 7). A plastic cuvette stirrer (5-in. long with a flat rounded edge) was used to quickly and with moderate pressure scrape the coated wood panel perpendicular to the grain pattern all the way across the short length of the panel and back. This motion was repeated rapidly 10 times and then the panel was inspected for any scratching or marring. Ratings were assigned using the following scale: 0 = no scratching, 1 = mild scratching, 2 = moderate scratching (like blank), and 3 = severe scratching (worse than blank). This method was found to be more reproducible than a fingernail scratch test.

For blocking studies, coatings prepared according to the formulation in Table 4 and containing 0.3 wt% surface control additives were applied as 200 mm wet drawdowns on primed beech panels. The coatings were dried for 5 h at room temperature. Blocking conditions used were: 1.6 kg/cm2 with Osimeter, 20 h at room temperature, one hour recovering; two coated wood panels were positioned coating-to-coating.
Design of the New Surface Control Additives
With the ambitious goal of designing organo-modified polydimethylsiloxane surface control additives that could provide the required surface effects while being recoatable, synthetic efforts turned toward the variety of molecular structures achievable with this chemistry. Not only are linear, comb, and branched structures possible, but variations in the length of the siloxane backbone, the position of polyether groups (organo-modification), and the length and chemical nature of these polyether groups are also possible.
To meet all objectives of this study, several key molecular attributes would need to be realized. First, the polydimethylsiloxane segments would need to be large enough to drive the molecule to the coating–air and coating–substrate interfaces in order to provide substrate wetting and adhesion as well as the benefits for which surface control additives are typically used (slip, scratch resistance, antiblocking, etc.). Second, there would need to be a means of compatibilizing the siloxane surface control additive in coatings formulations. In the case of waterborne coatings, one can achieve this by designing a water-based emulsion containing the active surface control additive. However, a 100% active surface control additive that is compatible in both aqueous and nonaqueous systems requires the introduction of a high degree of organo-modification in order to impart this compatibility.
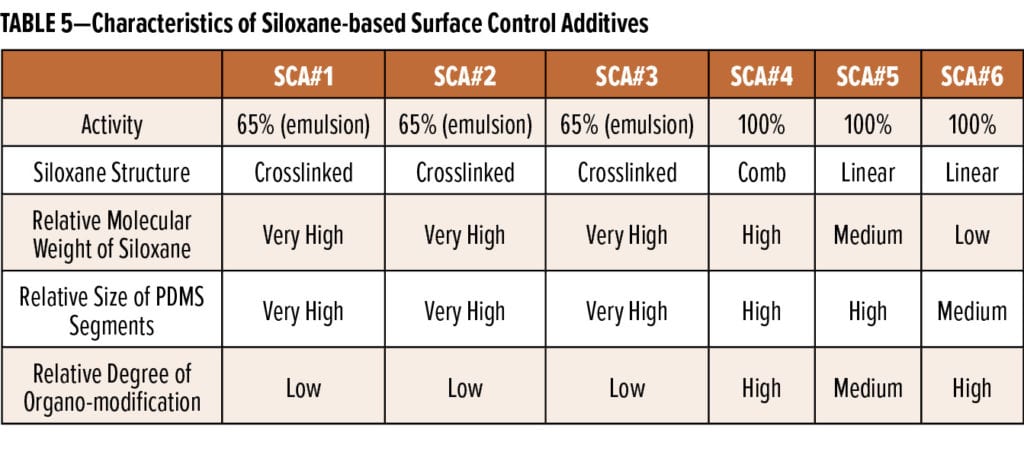
After many iterations within this synthetic space, four new surface control additives were developed; these include three siloxane emulsions (SCA#1, SCA#2, and SCA#3) as well as one 100% active siloxane (SCA#4). The characteristics of these four new materials are shown in comparison to two benchmark surface control additives (SCA#5 and SCA#6) in Table 5.
Evaluations of New Surface Control Additives in Waterborne Wood Coatings
To better understand the performance of these new surface control additives, they were evaluated in several different waterborne wood coating formulations. These included a commercial water-based oil-modified polyurethane clear semi-gloss as well as three internally prepared waterborne wood coating formulations—two clears and one pigmented—based on different self-crosslinking acrylic resins (see Experimental). It should be noted that the original objectives for each study differed; some were simple screens of the new additives with minimal comparison to other surface control additives, while others were more thorough comparative studies. As a result, SCA#5 and SCA#6 were not evaluated in all systems and some of the surface control additives were evaluated at fewer use levels than others.
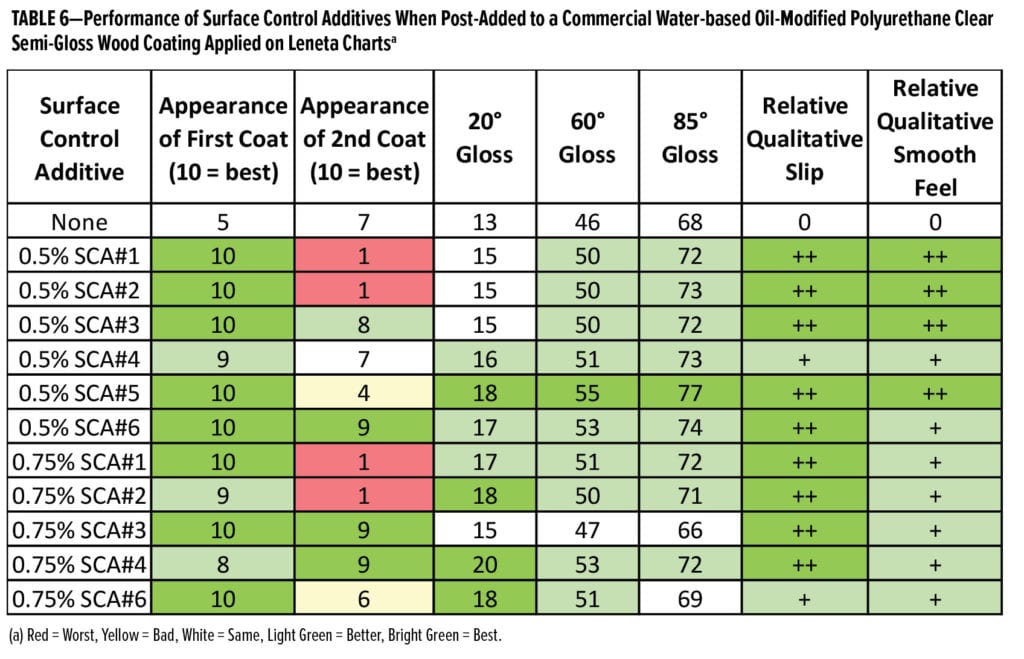
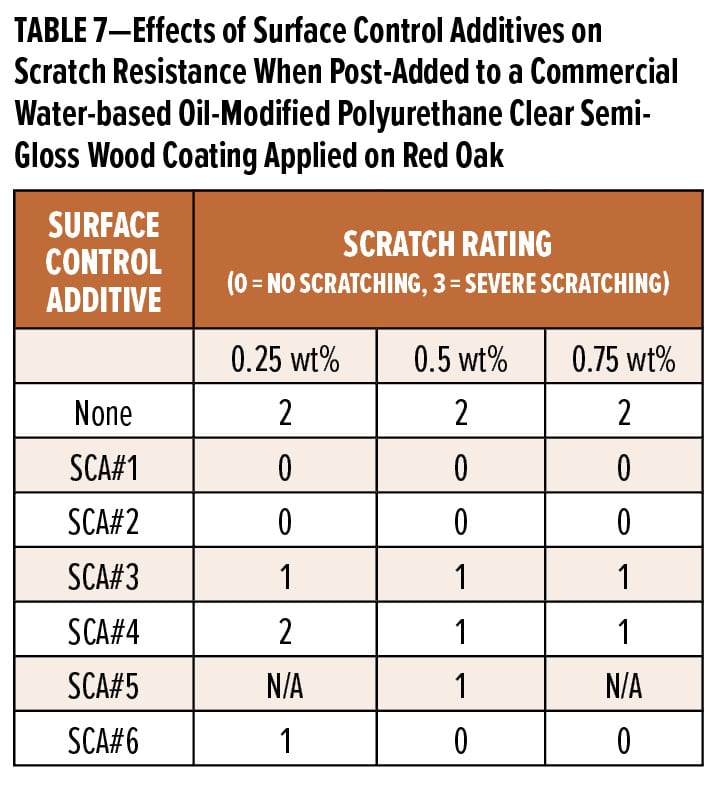
The first study undertaken was a screen of the surface control additives at 0.25 wt%, 0.5 wt%, and 0.75 wt% as post-additions to a commercial do-it-yourself (DIY) wood coating. Results for coatings applied to Leneta charts are shown in Table 6 and those for coatings applied on red oak are shown in Table 7. Clearly, all the surface control additives at either 0.5 or 0.75 wt% significantly improve the appearance of the initial coating; however, SCA#1 and SCA#2 impart considerable hydrophobicity to the coating surface and the appearance of the second coating layer is very poor, resulting in moderate dewetting. All the surface control additives increase the gloss as well as the relative qualitative slip and smooth, slippery feel. They all improve scratch resistance of this coating when post-added at 0.5 wt%, and SCA#1 and SCA#2—the most incompatible additives—provide the greatest improvement in scratch resistance. Overall, at 0.5 wt%, SCA#6 provides more benefits than the other surface control additives in this formulation; it increases gloss, slip, and scratch resistance of the coating while rendering it recoatable. SCA#3 and SCA#4 at higher use levels also improve the coating’s recoatability.
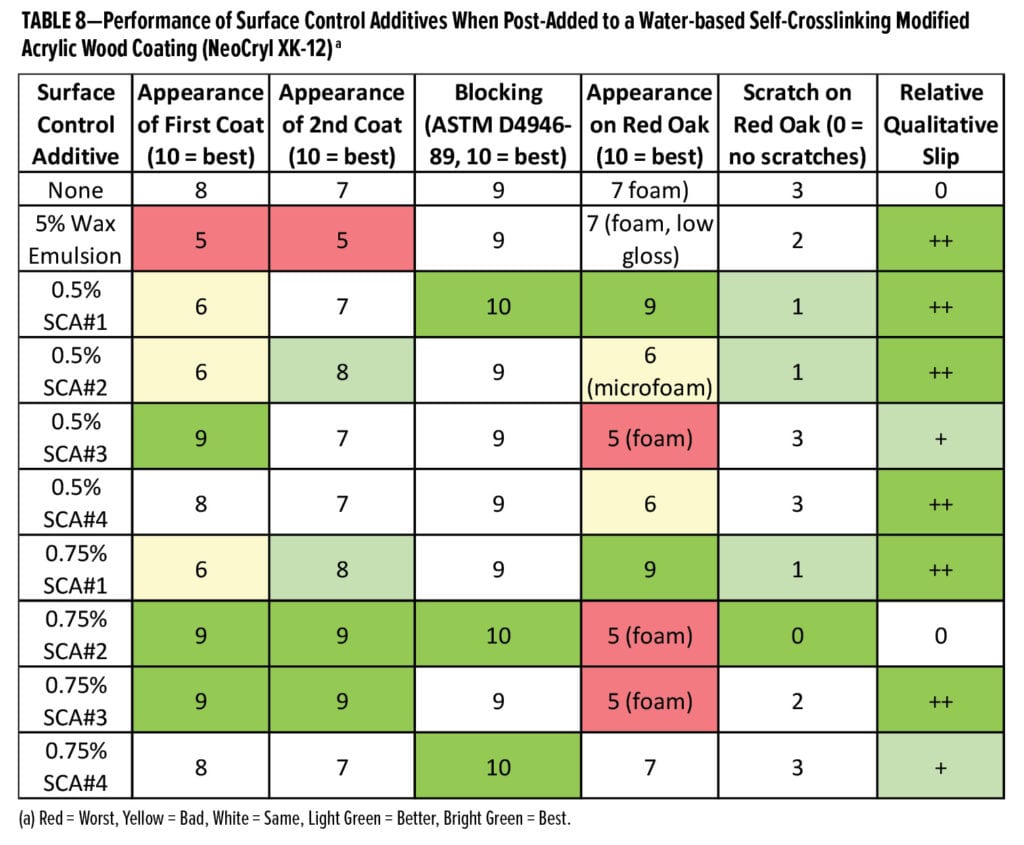
The second formulation studied was a water-based self-crosslinking modified acrylic clear wood coating based on NeoCryl XK-12 (Table 1). Results of post-adding 0.5 wt% and 0.75 wt% of the surface control additives to this coating are shown in Table 8. At 0.75 wt%, SCA#2 outperforms the other surface control additives with regard to improving blocking and scratch resistance; however, it seems to introduce more foam to the formulation. SCA#1 provides the best appearance when the coating is applied on red oak, and it provides a moderate improvement in scratch resistance at both 0.5 wt% and 0.75 wt%. The 100% active organo-modified siloxane SCA#4 appears to be too compatible in this coating formulation; therefore, it is not able to significantly improve scratch resistance of the coating when used at use levels up to 0.75 wt%.
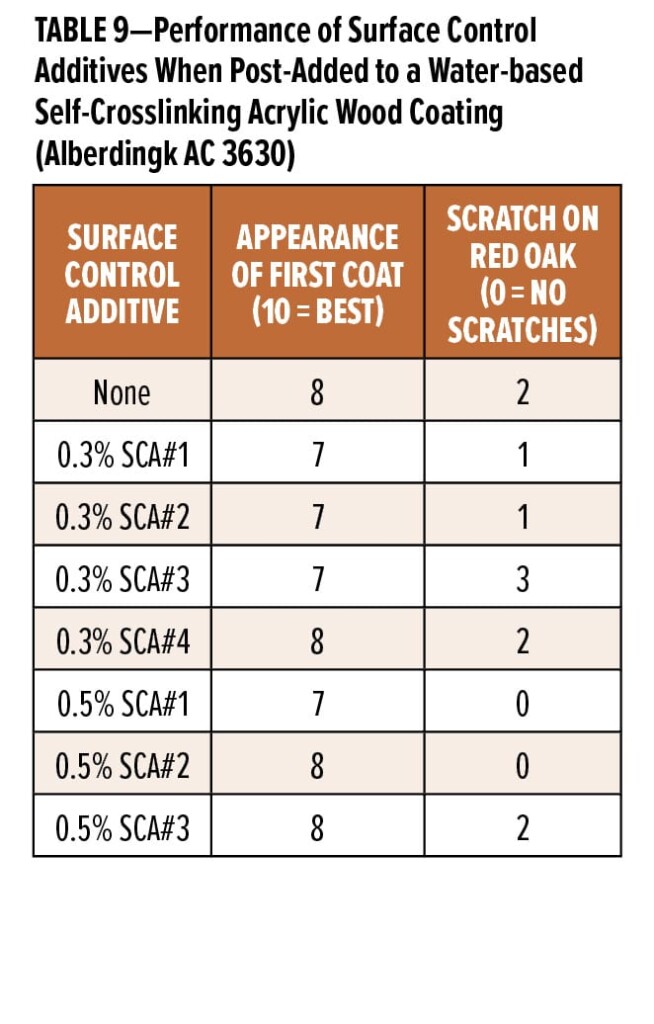
The third coating formulation studied was a water-based self-crosslinking modified acrylic clear wood coating based on Alberdingk AC 3630 (Table 2). Results of post-adding 0.3 wt% and 0.5 wt% of the surface control additives to this coating are shown in Table 9. Again, 0.5 wt% SCA#1 and SCA#2 provide the best scratch resistance, while SCA#3 hurt scratch resistance at 0.3 wt% and did not improve it at a 0.5 wt% use level. While SCA#4 was only tested at 0.3 wt% (the same actives level as was delivered by using 0.5 wt% of the three emulsions), it did not improve scratch resistance at this use level.
The final coating studied was a water-based pigmented acrylic wood coating based on Acronal LR 9014 (Table 4). Figure 8 shows the results of blocking tests performed using primed beech panels that had been coated (200 mm wet) using coatings containing 0.3 wt% active surface control additives. The Competitive SCA is an ultra-high molecular weight silicone dispersion in water that is promoted as a slip and antiblocking agent for waterborne wood coatings; it was used at 0.5 wt% in order to deliver 0.3 wt% actives on total formulation.
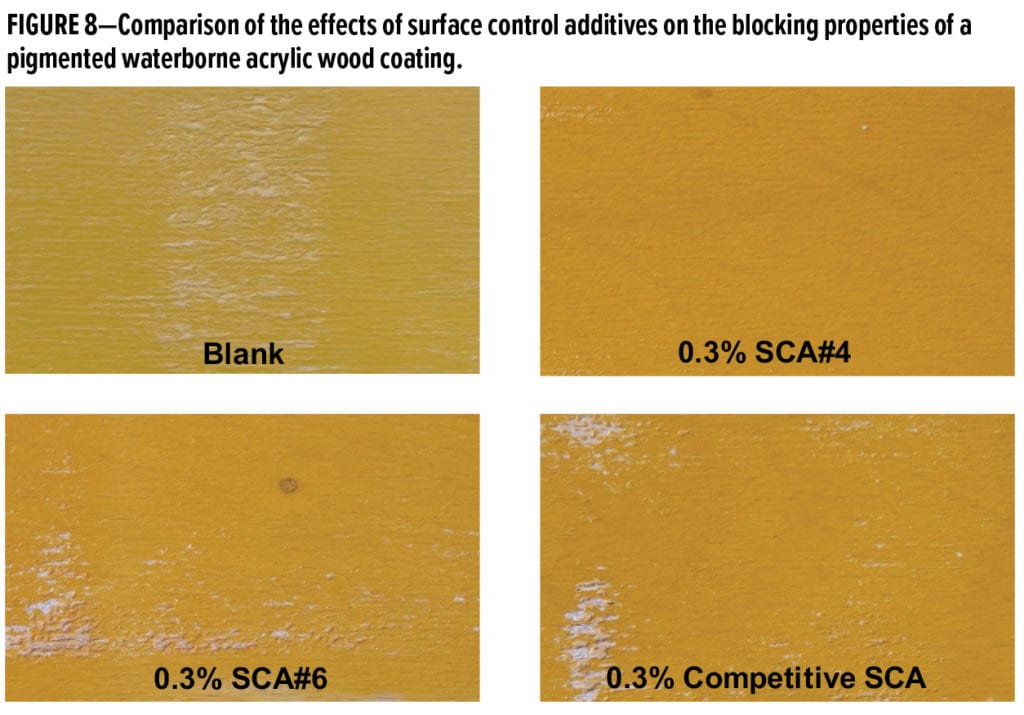
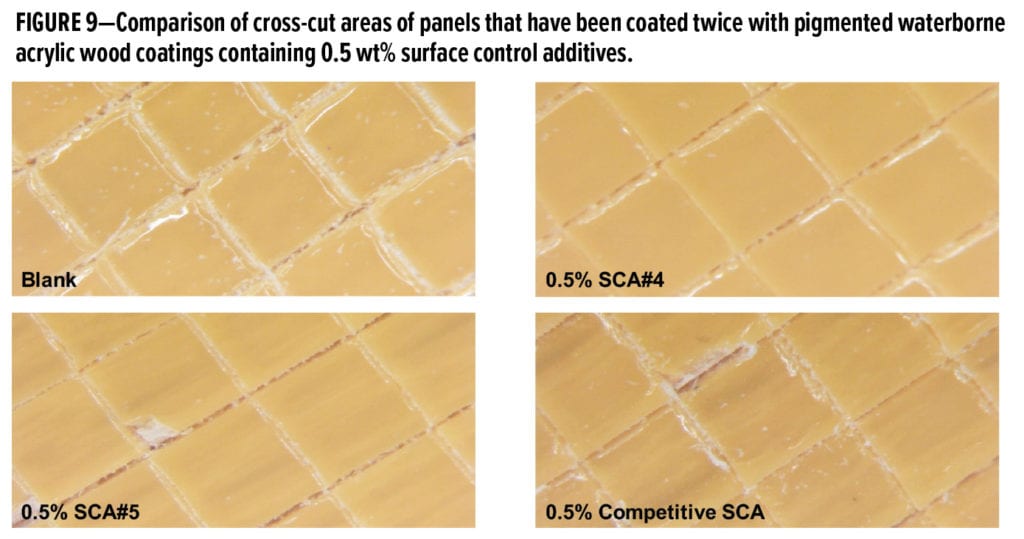
The effects of the new 100% active organo-modified siloxane surface control additive SCA#4 on recoatability in this pigmented water-based acrylic wood coating were also studied. A comparison of the cross-cut adhesion test panels is shown in Figure 9. At 0.5 wt%, SCA#4 shows excellent recoatability and no delamination of the second coating layer. However, the panels prepared using the coatings containing 0.5 wt% SCA#5 and 0.5 wt% Competitive SCA both show some delamination in regions near the cross cuts.
Discussion
This article has uncovered a few findings that should be mentioned. First, SCA#5 is extremely hydrophobic and proved to be quite incompatible in the two waterborne formulations in which it was evaluated. While SCA#5 can be used to provide a high-surface slip and silky feel, it is difficult to recoat with a waterborne coating; therefore, it might be more easily used in waterborne formulations that are intended as topcoats or overprint varnishes, and it is probably very advantageous in solvent-based and high solids coatings where its oleo-phobicity should render it very surface active. If a coating that contains SCA#5 requires recoating with a second waterborne formulation, the second coating should include an organo-modified siloxane designed to provide anticratering benefits. Also, intercoat sanding to improve adhesion and surface appearance should be considered if possible.
The more compatible 100% active organo-modified surface control additives, SCA#4 and SCA#6, can perform well—being sufficiently compatible with and providing significant surface benefits—in certain waterborne formulations. SCA#4 appears to be slightly more compatible than SCA#6 in the commercial oil-modified polyurethane wood coating, and SCA#4 showed better slip and feel at higher concentrations while SCA#6 provided better scratch resistance. With that said, SCA#6 was probably too compatible in the pigmented water-based acrylic wood coating because it was unable to significantly improve blocking. SCA#4, on the other hand, was able to provide antiblocking when used in the same formulation.
A comparison of the three new siloxane emulsion surface control additives has shown that, in general, SCA#1 appears to be most incompatible, SCA#2 a bit more compatible, and SCA#3 the most compatible in the waterborne coatings studied here. The additive that performs “best” in a formulation will really depend on what specific benefits are needed. For example, in a coating that employs a binder that has excellent blocking properties but is susceptible to scratching, a surface control additive that improves scratch resistance but has less of an impact on blocking might be the ideal choice. Other properties such as foam stabilization and anticratering properties may become important in coatings that require perfect mirror-like surfaces. Therefore, the formulator should consider several different surface control additives in order to identify the optimum one for the formulation under development.
Conclusions
Clearly there is no “universal” surface control additive. Each formulation and the application in which it will be used have different demands and the compatibility and properties of a surface control additive need to be matched to the specific system and its performance needs. The ideal surface control additive should provide the optimal balance between system compatibility and interfacial activity that minimizes issues and maximizes the benefits that these powerful tools can provide. Improvements in surface properties, including scratch and abrasion resistance, blocking behavior, and slip and haptic qualities, can be significantly enhanced with siloxane surface control additives. While compatibility and recoatability are significant concerns with this additive chemistry, these concerns can be readily overcome with a properly designed product.
The work described in this article highlights the fact that surface control additives can be engineered to provide a range of benefits in waterborne coating formulations. Of the four new surface control additives introduced in this work, two of the emulsions of crosslinked, high molecular weight polydimethylsiloxanes (SCA#1 and SCA#2) were found to provide very good scratch resistance while being less compatible in the water-based wood coatings. Interestingly, the third siloxane surface control additive emulsion (SCA#3) proved to be more compatible, resulting in very good surface appearance and haptic feel but little scratch resistance in the four wood coatings used in this study. While probably not the ideal characteristics for a wood coating, these properties are sought after in other coatings applications like leather coatings and graphic arts. The new 100% active organo-modified siloxane has good compatibility in the waterborne wood coatings studied as well as in other coating formulations. As a result, coatings containing SCA#4 may be recoatable and properties like antiblocking may be achieved in certain systems.
Clearly, siloxane-based surface control additives offer the formulator useful tools that can be employed to create high-performance coatings. This work has merely scratched the surface of what is undoubtedly an area that deserves further exploration, and additional fundamental studies to better elucidate chemical structure-property relationships are warranted.
References
- Owen, M.J., “Interfacial Activity of Polydimethylsiloxane,” in Surfactants in Solution, 6, Mittal, K.L. and Botherel, P. (Eds.), New York: Plenum Press, 1557–1569, 1986.
- Hill, R.M., “Siloxane Surfactants,” in Specialist Surfactants, Robb, I.D. (Ed.), London: Chapman & Hall, 143–168, 1997.
- Hill, R.M., Silicone Surfactants, New York: Marcel Dekker, Inc., 1999.
- The Big TEGO: Products—Services—Data Sheets, 4th ed., Evonik Industries AG, Essen, Germany, 68–75, 2014.
- Chen, H.H., “Scratch Resistant Low Friction/Low Surface Energy Coating for Silicon Substrate,” J. Appl. Polym. Sci., 37, 349–364 (1989).
- Liles, D.T., “The Fascinating World of Silicones and Their Impact on Coatings: Part 1,” CoatingsTech, 9, No. 4, 58–66 (2012).
- Liles, D.T., “The Fascinating World of Silicones and Their Impact on Coatings: Part 2,” CoatingsTech, 9, No. 5, 34–46 (2012).
CoatingsTech | Vol. 17, No. 3 | March 2020
