This paper will compare the advantages and disadvantages of different additive chemistries used to prevent defects caused by dewetting.
By , Evonik Corporation
Many surface defects, such as fisheyes, edge pull, and retraction are caused when the liquid film dewets after application. Application of the coating by brush, roller, or spray may effectively force wetting and spread the film across the substrate, but defects may form soon after application. There is competition between the hydrodynamic inertia of the applied film and the interfacial tension forces that can cause the coating to dewet or retract.
Additives can be used to prevent these defects by reducing the interfacial forces that drive retraction. However, with many different additives to choose from, that may also cause unwanted side effects, the formulator can find additive selection difficult. This paper will compare the advantages and disadvantages of different additive chemistries used to prevent defects caused by dewetting.
Introduction
The role of surface tension in the formulation and application of surface coatings has been acknowledged for many years. The surface tension of the coating is important to its ability to wet a surface, and many surface defects can be attributed to poor wetting or surface tension gradient driven flow.1,2 These include crawling or edge retraction, craters, fisheyes, orange peel and leveling patterns, picture framing, and poor recoatability.
Wetting is the displacement of one fluid (or gas) by another at a solid interface. It reflects the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. When a drop of liquid is placed on a solid surface, it forms a sessile drop in the shape of a sphere sectioned by the surface3 with a discrete and measurable contact angle between the sphere and the surface at the three-phase contact line (Figure 1). Thomas Young observed that there is an “angle of contact” for every solid/liquid pair and observed that this concerned a balance of forces that was later expressed in equation form where γsv, γLv and γsv are the surface free energies (not forces) for the solid-vapor, liquid-vapor, and liquid-solid, respectively. Wetting deals with all three phases of matter: gas, liquid, and solid.
FIGURE 1 Contact angle at equilibrium.
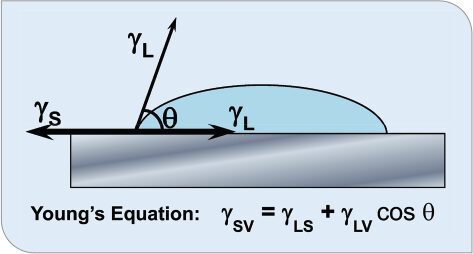
Young’s equation describes the situation at equilibrium, where the forces are equally balanced. Gutoff and Cohen describe that for spreading (wetting) to occur, “the forces to the left must be stronger than those to the right.”4 This leads to the well-known, and still applicable, rule of thumb that the surface tension of the liquid and the interfacial tension between the liquid and solid should be less than the surface energy of the coating. That said, Gao and McCarthy’s “Wetting 101” article highlights the limitations of this simplistic treatment and the need for better terminology regarding this complex subject.3 Unfortunately, only two of the four parameters in this equation can be measured experimentally and these measurements have limitations and require skilled interpretation.5 More critically, in real-world situations of non-ideal surfaces, the substrate surface roughness and inhomogeneous surface energy play a vital role in wetting, film stability, and dewetting.
When a liquid first contacts a surface, the initial contact area with the substrate is created by the forces applied. In the simple case of a droplet hitting a surface, the initial contact area is influenced mostly by the hydrodynamic forces applied during impact on the substrate—particularly the size, velocity, density, and kinematic viscosity of the droplet6-8—surface tension acts as a restraining mechanism. This can be visualized with high-speed photography, where a droplet of water initially spreads rapidly on a hydrophobic surface before retracting. When the surface tension of the droplet is changed, using different surfactants, the behavior of the droplet on impact and after also changes.
When coatings are applied, the initial wetting of a substrate occurs through the force used to apply the coating to the surface. Figure 2 shows the application of an overprint varnish over an oil-based lithographic ink. The drawdown application ensures that the varnish fully covers the hydrophobic surface; however, less than a second after the drawdown is completed, dewetting begins rapidly as the cohesive forces within the liquid cause the liquid to contract. This highlights how liquids may retract or dewet after application, and this is usually what happens when a coating is applied. The force of the application method may ensure that a surface is initially covered by a liquid but does not ensure that the liquid will remain in place.
FIGURE 2 Dewetting of an overprint varnish over lithographic ink after application.

Continue reading in the May-June digital issue of CoatingsTech
