By Cynthia A. Gosselin, Ph.D., The ChemQuest Group
First published in 1939, one of the oldest specifications in the Committee D01 arsenal is ASTM B117 Standard Practice for Operating Salt Spray (Fog) Apparatus. It is still in active use today in a wide variety of markets including automotive, marine, aerospace, and building construction to evaluate, using a standardized approach, the comparative durability and performance of substrates in corrosive environments. This is despite some experts suggesting that the test is outdated and convenient rather than scientifically rigorous.
Corrosion is a significant challenge for any industry that uses metallic substrates. Protective metal coatings and structural integrity can be adversely affected by corrosion. Salt spray testing is a method designed to provide a controlled corrosive environment using a salt fog chamber to evaluate the corrosion resistance of metallic and nonmetallic coated metal substrates.
On the surface (no pun intended), this test is easy to perform. It is quick, cost effective, and laden with decades of historical comparative data on a wide variety of substrates. Samples are placed in a sealed chamber within which is generated a continuous fine 5% neutral aqueous sodium chloride mist onto samples placed at a 45° angle. Temperature, humidity, pH, and airflow are all specified within the practice and regulated within the test chamber to provide as consistent an exposure environment as possible. Exposure hours can vary from 24 hours to over 1,500 hours, depending upon the level of corrosion resistance required.
Here begins the controversary about the real influence of salt spray testing. The practice clearly states that this is a test that is designed to “produce relative corrosion resistance information.” However, over the decades, a common misconception has grown that the test can predict real-world product service life. Often customers self-correlate hours to salt spray failure with product life and even try to reject products or extract warranty based on hours to failure.
Most coating and corrosion experts dispute this premise. Real-world service life includes an uneven cycle of humidity, UV exposure, temperature variations, pollutants, cleaning solutions, vibration, and other environmental factors that are not captured in continuous salt fog chambers.
Rather than predicting service life, salt spray testing is well suited for comparative evaluations of a host of coatings, plated finishes, pretreatment efficacy, pre- and postcoated sheet and formed parts, and manufacturing quality in a controlled environment.
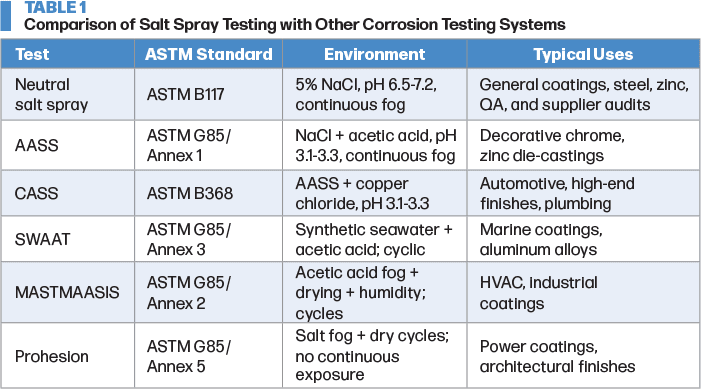
If predictive testing is required, it is necessary to augment neutral salt spray (NSS) testing by expanding into cyclic and real-world testing. Those tests are also ASTM standards. Included are acetic salt spray (AASS), copper accelerated salt spray (CASS), acidified seawater cycles (SWAAT), acidified acid fog/drying/humidity cycles (MASTMAASIS), and prohesion (wet fog/dry off cycles). Continuous testing (NSS, AASS, CASS) in constant fog environments is useful for benchmarking and comparative testing, but poor real-world simulations. Cyclic tests (SWAAT, MASTMAASIS, prohesion) that alternate wet/dry/humid and wet phases more closely simulate real-world environments. Table 1 further compares the various corrosion tests.
Salt spray testing is not a dinosaur approaching extinction. Rather, this 86-yearold test provides a foundational benchmark for product behavior in a corrosive environment. It is a consistent, reproducible method for comparative benchmarking evaluation of corrosion performance under continuous corrosive chamber conditions, useful for monitoring process quality and consistency, evaluating plated finishes, supporting root-cause failure analysis, benchmarking coatings, and pretreatment efficacy. However, the caveat is that the test does not provide real-world long-term performance as it does not include other environmental influences. Predictive testing should be augmented by cyclic and/or real-world environmental testing.
Cynthia A. Gosselin, Ph.D., is director at The ChemQuest Group, www.chemquest.com. Email: cgosselin@chemquest.com.
