Matthew M. Sumpter, Franklin I. Leal, Mingbo He, and Daniel J. Weinmann, Westlake Epoxy
Epoxy resins have been used for decades in applications demanding excellent adhesion and corrosion resistance, but historically, they have not been suitable for coatings where ultraviolet (UV) resistance is needed. A direct-to-metal (DTM) coating, comprised of a novel modified cycloaliphatic epoxy resin combined with a lower yellowing amine curing agent, was designed to deliver superior performance in corrosion resistance and adhesion. Additionally, this epoxy resin system offers improved gloss retention and color stability compared to standard epoxy coatings.
Both the resin and curing agent are optimized to minimize dry time while maximizing hardness development. The relationship between epoxy-amine stoichiometry and coating performance was evaluated to further improve the system. Comparative testing with a commercially available epoxy-polyamide DTM coating showed that the novel epoxy system performed well against the competitive material while significantly improving the UV resistance, as determined by gloss and color retention.
Introduction
Brief History of Epoxy Coatings Relevant to DTM Applications
For decades, it has been widely known and accepted that coatings based on aromatic epoxy resins (typically based on bisphenol A) and cured with amines perform poorly in exterior or UV-exposed applications.1 The literature on potential mechanisms causing this poor performance is extensive, but essentially the main arguments for consideration here involve photodegradation and microstructure rearrangement that lead either to discoloration through chromophore generation (understood as yellowing), loss of gloss, or both.2,3 Were it not for these mechanisms, epoxy coatings would likely have little to no need of further development for industrial DTM applications since the remainder of properties achievable with typical epoxy coatings is generally desirable, especially resistance to corrosion and chemicals. Often, a formulator’s first choice for improving the known deficiencies of standard epoxy resin would be to mitigate UV sensitivity with additives such as hindered amine light stabilizers (HALS) and ultraviolet absorbers (UVAs), and in many cases improved results are achieved, but this approach is more of an incremental improvement than stepwise.4
With protective industrial coatings, application time and labor are particularly expensive, so the more layers of coating that are needed to satisfy the technical requirements, the higher the project costs will be. It is apparent that a single-layer DTM application that can meet all customer needs offers great value for formulators and applicators. Current product offerings tend to be either DTM epoxy systems with excellent corrosion and chemical resistance, but higher yellowing, chalking, and gloss loss that are not suitable for exterior or UV-exposed applications, or DTM options based on urethane or acrylic technology that mitigate the UV resistance issues of epoxy while also giving up other desirable characteristics, rendering them unfit for many protective DTM applications. To satisfy an unmet market need, new coatings technology is needed in the form of a lower yellowing epoxy resin for industrial DTM applications that can perform in both UV and corrosion resistance.
Baseline Performance of Common Epoxy Resins
Before developing a novel epoxy resin for industrial DTM applications, it was deemed necessary to evaluate the relative coating performance of common epoxy resins to define the deficiencies. To simplify, future references to “Standard Epoxy” can be understood to denote a typical resin blend (epoxy equivalent weight ∼ 188 g/mol) consisting of diglycidyl ether of bisphenol A (DGEBPA), diglycidyl ether of bisphenol F (DGEBPF), and an epoxy-functional reactive diluent, and any reference to “Cycloaliphatic Epoxy” represents the diglycidyl ether of hydrogenated bisphenol A. For curing agents, “Standard CA” is a typical isophorone diamine (IPDA) adduct with DGEBPA and other formulated components, typical of many products in the epoxy curing agent market, while “LY CA” represents a modified cycloaliphatic amine composition, for improved lower yellowing performance.5
Unless otherwise noted, all coatings tested were applied via drawdown (DFT of 3-4 mils) and cured at 25 °C, 50% relative humidity onto the respective substrates needed for the various tests performed. For comparing UV resistance of Standard Epoxy and Cycloaliphatic Epoxy with both Standard CA and LY CA, a cyclic QUV-A test was performed according to ASTM G154, Cycle 2 (8 h at 60 °C UV exposure, 4 h condensation at 50 °C) on coatings applied to Q-PANEL A-36 substrates. Figure 1 shows that there is a significant decrease in observed color change when using Cycloaliphatic Epoxy versus Standard Epoxy, as expected.
FIGURE 1 ΔE vs time in cyclic QUV-A for clearcoats of various epoxy resins and curing agents.
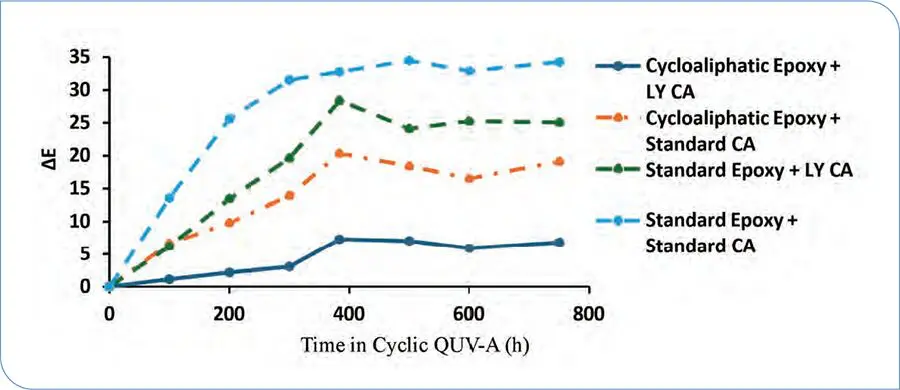
While the shift in yellowing resistance from Standard Epoxy to Cycloaliphatic Epoxy is greater than that from Standard CA to LY CA, to achieve long-term UV resistance in a lower yellowing DTM system, both resin and curing agent should be capable of superior yellowing resistance. Although coatings made with Cycloaliphatic Epoxy display good UV resistance, this comes with a significant decrease in cure speed (observed by users as an increase in dry times). To establish the performance gap of Cycloaliphatic Epoxy cured with LY CA versus Standard Epoxy cured with LY CA, circular drying times were determined (following ASTM D5895) on coatings applied to Form 1A Penopac Charts. In addition to the earlier systems, a modified cycloaliphatic epoxy resin designed for lower yellowing, faster cure concrete coatings (now referred to as Accelerated Cycloaliphatic) was included to represent a lower yellowing control with acceptable drying time. All systems were formulated according to the recipe in Table 1 with resin, curing agent, and pigment loadings adjusted appropriately to yield a High Gloss White Enamel with an epoxy:amine stoichiometry of 1:1. Circular dry times are listed in Table 2.
Continue reading in the January-February issue of CoatingsTech
