By , , , and , Arkema, Inc.
For many years, aqueous hybrid dispersions of poly(vinylidene fluoride) (PVDF) and acrylic resins have been formulated into waterborne topcoats because of their exceptional weatherability and ability to meet stringent volatile organic compound (VOC) requirements and sustainability targets. For applications requiring strong chemical resistance, hydroxy-functional PVDF-acrylic dispersions are available which allow formulators to build resistance properties with isocyanate crosslinking. However, isocyanate not only presents respiratory hazards to paint applicators, but it can cause microfoaming in the resultant films due to CO2 generation from the reaction of isocyanate with water. Recently, new one-component self-crosslinkable PVDF-acrylic hybrid latexes were successfully developed, and can be formulated below 100 g/L VOC. Detailed properties, such as detergent resistance, chemical resistance, and dirt pick-up resistance, in target applications such as architectural façade coatings and protective coatings are presented.
Introduction
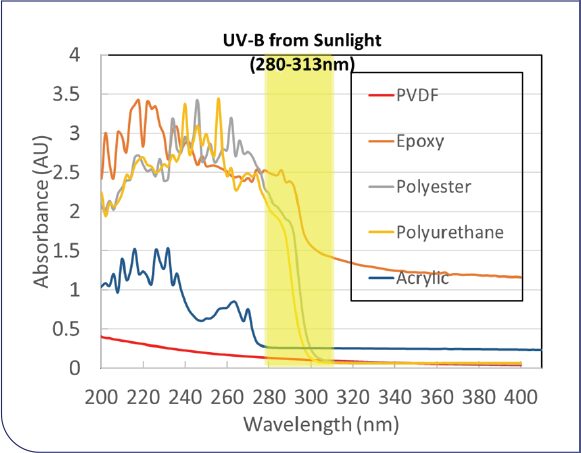
Poly(vinylidene fluoride) (PVDF)-based topcoats1 have been used for decades on monumental buildings around the world, and they have become the gold standard for decorative property durability and substrate protection. This performance is attributed to PVDF’s outstanding UV and chemical resistance, and its good barrier properties against moisture and oxygen. The superior UV and chemical resistance of PVDF, relative to other common coating polymer chemistries, is demonstrated by its extremely low absorbance in the UV, as can be seen in Figure 1. The UV absorbance spectra were normalized to 25 μm dry film thickness. PVDF copolymer with hexafluoropropylene (HFP) comonomer was used in this study.
FIGURE 1: UV absorbance spectra of PVDF and other polymer chemistries. UV-B band (280-313 nm) from sunlight is highlighted in yellow.
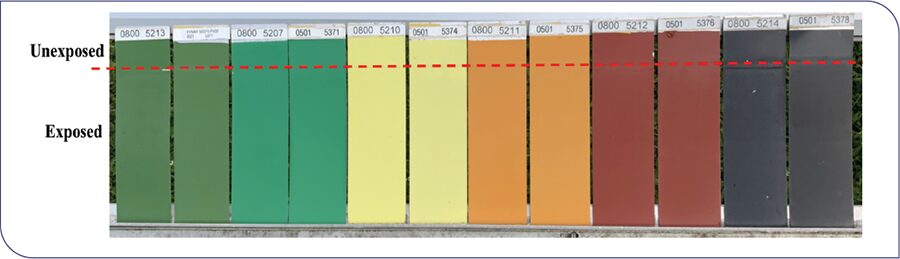
PVDF-based coatings have historically been limited to factory-baked coatings on metal because of the need for a high-temperature bake (230-250 °C) to alloy the polymer components. These baked PVDF coatings, commonly used as topcoat finishes on monumental buildings and other architectural applications, typically contain a blend of 70-80 wt % PVDF resin with 20-30 wt % of a miscible acrylic resin. Around the year 2000, water-based hybrid resin technology became commercially available,2 combining PVDF copolymers and acrylic resins in pre-alloyed form.3 The new water-based technology allows for the application of durable PVDF-based coatings under field-applied and low-temperature bake OEM conditions, with dramatically lower levels of emitted volatile organic compounds (VOCs). Figure 2 shows some South Florida weathering panels for a commercial waterborne PVDF-acrylic binder, after 20 years of exposure. Minimal visible differences can be noted between the unexposed and exposed areas of the panels.
FIGURE 2: Twenty years’ South Florida field exposure weathering panels of one-component PVDF-acrylic-based waterborne coatings (as shown in left for each color) vs legacy solventborne baked PVDF-acrylic coatings (right for each color). Panels are exposed at 45° South-facing. The substrates of these panels are chromated aluminum Q-Panels (AL-412 from Q-Lab Corporation).
More recently, hydroxyl-functional PVDFacrylic hybrid products in this class were developed, which could be used with crosslinkers such as water-reducible polyisocyanates.4,5 These systems show enhancements in certain key properties such as early hardness, barrier properties, solvent resistance, and adhesion, with performance contributions coming both from the entangled polymer network and from the network formed by crosslinking reactions.6
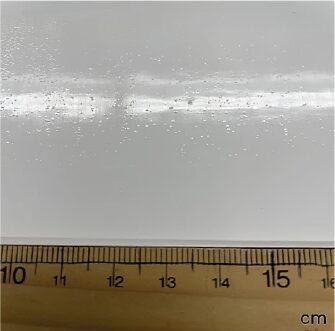
However, similar to other two-component systems with isocyanate crosslinking, isocyanate not only poses respiratory hazards and carcinogenic potential to paint applicators, but it can also cause microfoaming in the resultant films due to CO2 generation from the reaction of isocyanate with water in the paints. This is particularly noticeable when applying paint with a brush or roller outdoors (Figure 3).
FIGURE 3: Microfoaming issue with two-component waterborne paint with isocyanate crosslinking.
In this article, we present studies for developing a one-component selfcrosslinkable PVDF-acrylic hybrid latex and waterborne formulations based on this hybrid latex for architectural façade coatings and protective coatings.
Experimental
Synthesis of Self-crosslinkable PVDF-acrylic Hybrid Latex
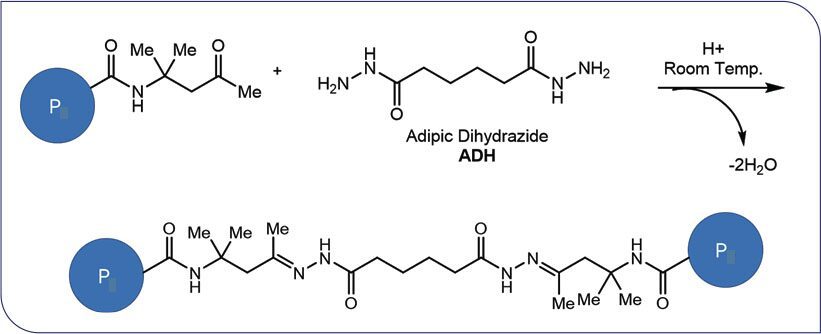
A two-stage process was used to make the PVDF-acrylic hybrid latex.7,8 PVDF latex was made in the first stage through emulsion polymerization of vinylidene difluoride monomer and an optional HFP comonomer in a pressure reactor. The second stage of the polymerization process, also called the acrylation stage, incorporated the acrylic monomers into PVDF latex to produce the intimate micromolecular mixture of PVDF and acrylic polymers. A wide variety of acrylic monomers with different Tg and functionalities can be chosen to tailor the coatings properties to the desired requirements. In this work, diacetone acrylamide (DAAM) monomer was introduced into the PVDF-acrylic hybrid latex along with other acrylic monomers and was copolymerized within the acrylic copolymers, creating well-dispersed pendant ketone crosslinking sites. Adipic dihydrazide (ADH) acted as a difunctional crosslinking agent, remaining partitioned in the water phase outside of the emulsion particles. ADH could be either added to the latex after emulsion polymerization was complete or during the paint formulation stage. Acrylic emulsions based on DAAM with ADH were initially nonreactive, ensuring emulsions maintained good long-term stability during shipping and storage. One-component keto-hydrazide self-crosslinking can occur at ambient temperature, facilitated by water evaporation during the film drying process and a simultaneous reduction in pH resulting from the loss of ammonia (a neutralization agent added in emulsion polymerization and paint formulation) (Scheme 1).
SCHEME 1: Keto-hydrazide self-crosslinking mechanism. P denotes the polymer chain.
Continue reading in the July-August digital issue of CoatingsTech
