By Greg Monaghan and Alex Pinnix, Specialty Polymers, Inc.
Most water-based architectural stain blocking primers typically have only moderate stain blocking performance, and they are designed to lock stains in the primer coat rather than block stains. This leads to a major performance difference compared to solvent-based primers. Since the stain is visible in the dry film when a bleeding substrate is coated with water-based primer, the painter cannot tell with any degree of certainty if the stain will migrate into the topcoat. Novel cationic water-based primers offer far superior stain blocking and are like solvent-based primers because many stains are blocked by the primer coat rather than locked into the primer coat. For these cationic primers, however, manufacturing procedures and raw materials are different from those typically used in conventional anionic architectural coatings.
Introduction
When painting, some types of stains can bleed from the substrate and discolor the topcoat. Tannins in wood, dye, and colorants from children’s washable markers, water, or smoke stain damage and staining at knots are particularly prone to bleeding stains and often require the use of special stain blocking primers.1,2
Although a variety of water-based stain blocking primers are on the market, these are best for blocking light tannin staining and light household stains. For more difficult to block stains like dark tannin stains on severely bleeding woods (redwood, cedar, or merbau), water damage or nicotine stains, often solvent-based primers or alcohol-based shellac primers are required.1 The solvent or alcohol-based primers have typical problems associated with solvent-based paints, however—strong odor during application, flammability, and lack of water cleanup. In addition, the tannins in wood can deactivate the driers used in alkyd primers giving a much slower dry. Shellac-based primers do not require driers and do not have slower dry on high tannin woods, but they are typically only recommended for spot priming in exterior applications because they are prone to cracking.1
Part of the reason that excellent stain resistance is difficult to achieve in water-based primers is that many of the common stains are either anionic or phenolic. They can be dissolved in alkaline water that forms the carrier in most conventional water-based primers, and the stains can be leached from the substrate into the primer coat. For this reason, many water-based primers use reactive pigments or salt solutions like zinc oxide, zinc, or zirconium ammonium carbonate,3,4 calcium barium phosphosilicate, or barium metaborate, which form cationic ions to complex with the anionic stains. The complexed stains are theoretically less likely to migrate from the primer coat into the topcoat.
Although theoretically effective, there are several reasons why this approach to formulating a water-based primer performs worse than solvent-based primers. The use of reactive pigments can lead to instability or loss of stain blocking efficiency if the cationic reactive pigments react with the anionic primer polymer. In addition, the film formation of the water-based polymers is often not as good as the solvent-based primers, leading to channels through the primer coat for the stains to reach the topcoat. Compared to water-based primers, solvent-based primers are also more effective at stain blocking because the carrier solvent does not dissolve the hydrophilic anionic stains, and a single coat of solvent-based primer will usually block the stains in the substrate, rather than locking them in the primer coat.
Many approaches have been attempted to improve the stain blocking performance of waterborne primers. Patents have been granted on the use of ground ion exchange resin,5 nano-particle size cationic clay,6 titanate coupling agents,7 monomers with strong acid functional groups,8 or amino silanes9 to help complex the tannins. Cationic water-based primers10 have also been reported to have very good tannin block resistance.
Cationic water-based stain blockers offer some significant advantages in that the anionic stains can react with cationic functional groups on the polymer itself, and because the low pH water of the cationic primer does not dissolve tannin stains as well as alkaline water in the anionic primers. In some cases, because of this reduced solubility of the stain in the primer coat, the stains do not bleed into the primer coat, and some cationic water-based stain blockers can give stain blocking performance almost as good as solvent-based primers. Water-based acrylic cationic polymers have been available for several years, however, a water-based cationic epoxy ester polymer11,12 formulated at low pH has been reported to give very good stain blocking like that of several solvent-based primers.13 This cationic epoxy ester does not require a drier so it has good drying over high tannin substrates that would deactivate the driers in conventional alkyd primers.
Despite their good stain blocking performance, some epoxy esters can undergo loss of adhesion through saponification on alkaline masonry14 or by contact with zinc in galvanized steel. In addition, paints based on the cationic epoxy ester are reported to crack and cold check on wood exposed to thermal cycling.15 Using a blend of cationic acrylic polymer with the cationic epoxy ester would be expected to reduce the saponification and cold checking significantly. A series of paints was prepared to evaluate blends of cationic acrylic with cationic epoxy ester for typical primer properties.
In preparing these cationic primers, primer ingredients must be selected so that they do not interact with the cationic polymer and prevent the cationic functional groups from interacting with the stains. The extender pigments used should not have an intrinsic high pH like nepheline syenite, wollastonite, or calcium carbonate. Silicas and clay extender pigments are closer to neutral pH in aqueous solutions16 and work well in these cationic primers. Silica or silica/alumina treated grades of titanium dioxide also work well while alumina treated grades of titanium dioxide have a high pH isoelectric point17 and can also tie up cationic sites on the binder. By the same token, the dispersants used should either be nonionic or cationic so that they do not react with the cationic sites on the polymer. Nonionic HEUR associative thickeners work well in these cationic paints, giving good films with fewer hydrophilic domains than HEC thickeners. The pH can be adjusted if necessary to the 5.0–6.0 range using a volatile acid like acetic or formic acid.
One additional potential issue is a tendency of these cationic primers to cause flash rusting if the primer is applied to sheetrock nail heads or they may cause corrosion inside metal cans if the lining has a defect. Many of the conventional flash rust additives like amines, sodium nitrite, or ammonium benzoate do not offer protection or are unstable at the pH 4–6 range common with these cationic primers. A nitrite-free liquid flash rust additive based on alkanolamine borate and phosphate salts18 has been found to be effective and stable in these low pH cationic primers, however.
Test Methods
Marker and ink stain blocking was tested by applying lines of black Sharpie permanent marker; black, blue, and red Papermate Profile ballpoint pens; red Papermate Flair; red and blue Crayola washable markers; and green Bic Briteliner 3n1 Highlighter to an unsealed Leneta card. Inks and markers were dried overnight, then primers were drawn down using a .003 Bird blade across the lines. Primers were dried for two hours, then a single coat of commercial polyvinyl acetate (PVA) flat wall paint was drawn down over the primer coat. After aging for 24 h, the individual lines were evaluated for bleed through on a visual 1–10 scale, with 10 being no bleed.
Tannin stain blocking was run by applying the primer to six-inch sections of 1 in. x 6 in. redwood decking panels at 350 ft2/gal. The primer was dried for four hours, then the sections were top coated with a commercial interior PVA flat paint. Panels were then immediately placed into a chamber at 80% relative humidity and 70°F for 16 h, then removed and allowed to dry. The sections were evaluated for tannin bleed by measuring Delta b* (yellowness/blueness) compared to the same paints drawn down on a white Leneta card. A fail control of two coats of PVA topcoat without primer was used to confirm the severity of the tannin bleed of the selected boards.
Wet adhesion to aged gloss alkyd and metal was run by applying the paints at 3 mil wet film thickness over four-week aged drawdowns of gloss alkyd (Valspar 4000 Alkyd Enamel #451858) or metal test panels (ACT hot dipped galvanized panels #G70 70U, Q-Panel A36 untreated aluminum, and Q-Panel R36 matte finish cold rolled steel). After aging for one week for the gloss alkyd or two days for the metal panels, the sections on each panel were tested for wet adhesion by soaking the panels for one hour, and then rating the blistering, the ease of peeling from the substrate at a knife cut, and the % removed by tape pull using Intertape CIC7080610 #51596 brand tape on a crosshatched section.
Sandability was evaluated by drawing the primers down using a 3 mil Bird blade on black vinyl scrub charts. The drawdowns were dried overnight, then the paints were sanded with 20 back-and-forth strokes using 200 grit sandpaper. Primers were rated for sandability by observing the dusting residue on the sandpaper. Paints were rated from 1–10 with 10 indicating the paint was easily sanded and had fine dust (no eraser-like residue).
Grain crack resistance was tested by applying two coats of the primers to yellow pine decking then subjecting the panels to thermal and water soak cycling. The painted panels are soaked for four hours, immediately placed in a freezer for 16 h, then placed in an oven at 120°F for four hours (this is one cycle). Panels were run for five cycles. Cracking was rated visually from 1–10, with 10 being no cracking.
Results
The washable marker and ink stain blocking of the primer coat alone or with a PVA topcoat applied over the primers are shown in Figures 1 and 2 and Table 2.

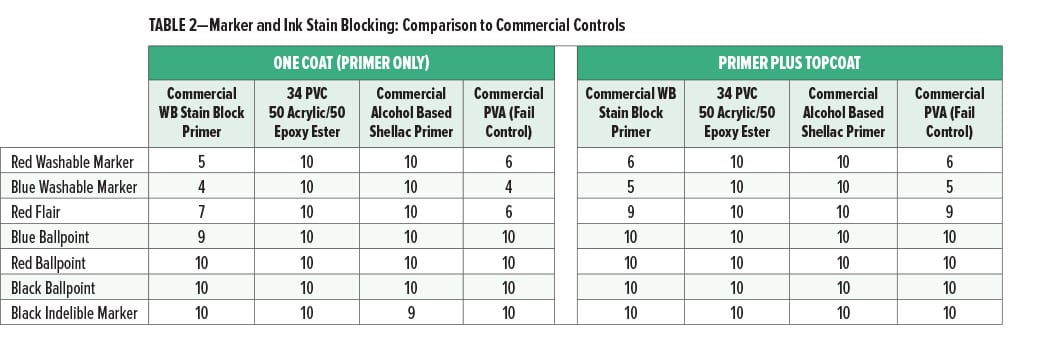
The washable markers and inks had no significant bleed into the experimental acrylic/epoxy primer coat (34% PVC, 50% acrylic/50% epoxy ester) and excellent bleed resistance in a primer plus topcoat system. Since the markers did not bleed into the primer coat, the primer may be a more effective barrier to the bleed of the markers and inks into the topcoat. The performance of the experimental primer for washable markers was better than the commercial water-based stain blocking primer and equal to that of the commercial shellac alcohol-based primer.
The marker and ink stain blocking for different ratios of acrylic to epoxy ester compared to the commercial water-based stain blocking primer is given in Figures 3 and 4 and Table 3.
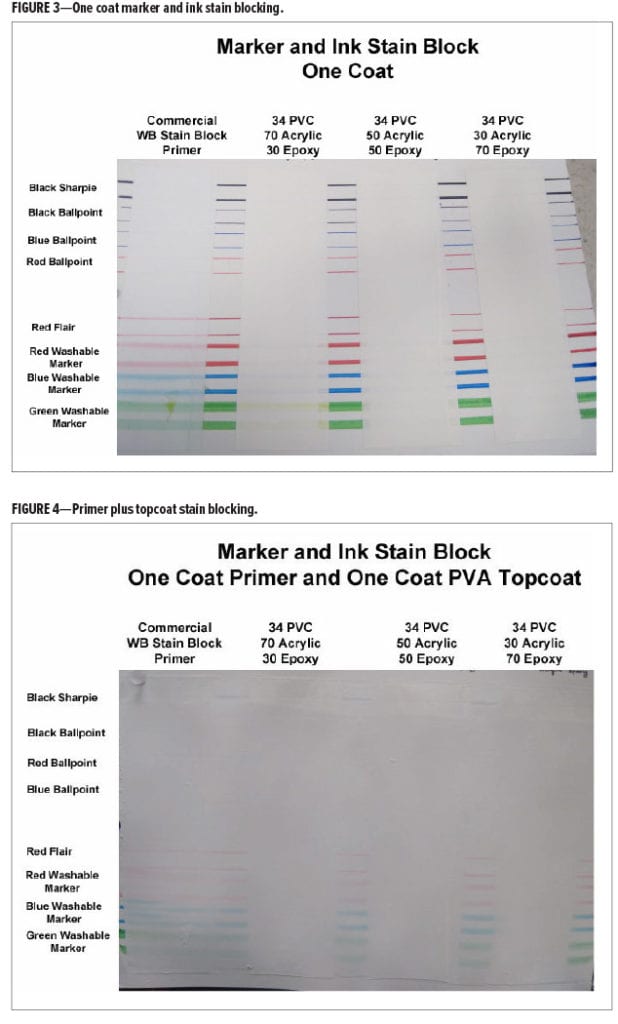
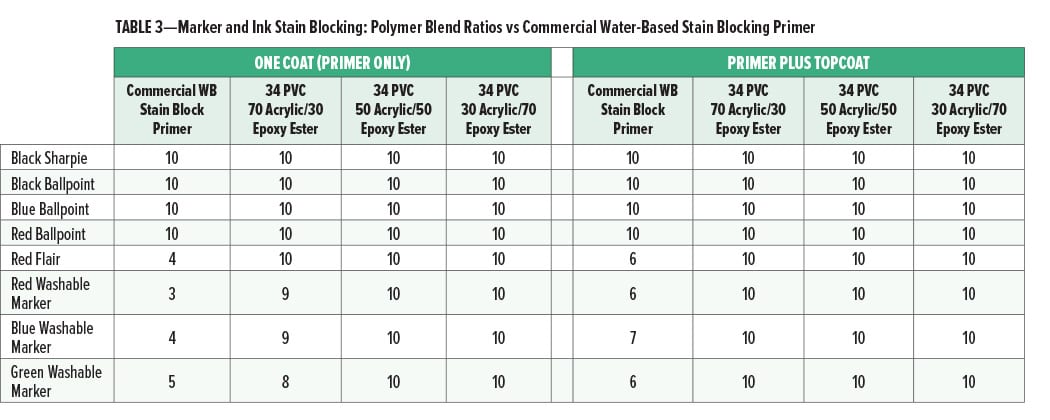
Although the topcoated cationic primers were all excellent for stain blocking, there was a difference in performance in one coat stain blocking. At a ratio of 70% acrylic and 30% epoxy ester there was a slight bleed of the washable markers into the primer coat, while the paints with the 50/50 and 30/70 acrylic to epoxy ester ratios had no bleed. This may indicate that at least 30% of the epoxy ester is necessary for optimal one coat stain block. While the use of the epoxy ester is necessary for one coat marker stain blocking, it is not clear from this study how the cationic epoxy ester is improving that property since it is significantly different from the cationic acrylic in several ways that could affect marker stain blocking. For example, the backbone hydrophobicity and molecular weight; the level of stabilizing surfactant; or the level, type, or availability of cationic functional groups on the epoxy ester relative to the cationic acrylic could all affect this property.
The marker and ink stain blocking at 30, 34, and 38% PVC (all at 50% acrylic and 50% epoxy ester) is given in Table 4.
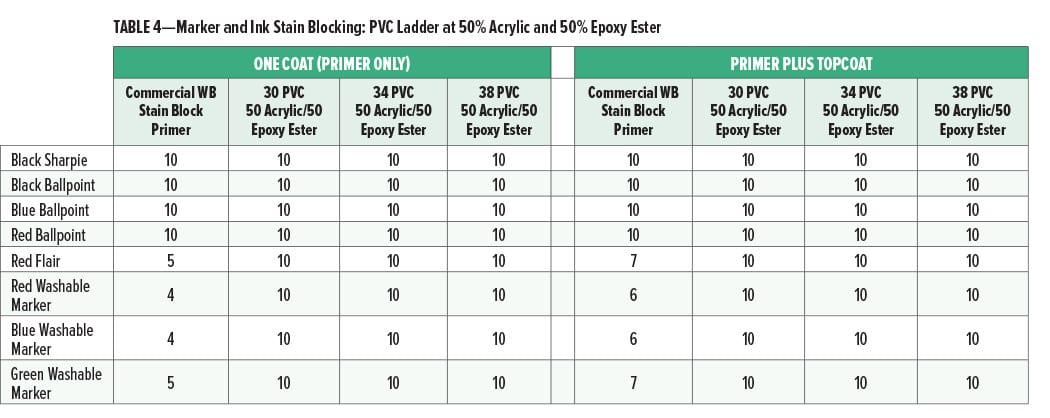
At 50% acrylic and 50% epoxy ester, the marker and ink stain blocking were very good for pigment volume concentrations up to 38%. Primer formulations which have good stain blocking performance at relatively high PVC may be lower cost than similar primers at lower PVC (and equal volume solids) and, therefore, more commercially acceptable.
The tannin stain blocking (reported as Delta b compared to a drawdown of the same paint on white Leneta card) on redwood decking for the primer coats at different ratios of acrylic to epoxy ester (34% PVC) is given in Figure 5 and Table 5. The redwood panel was topcoated with the interior PVA flat wall paint.
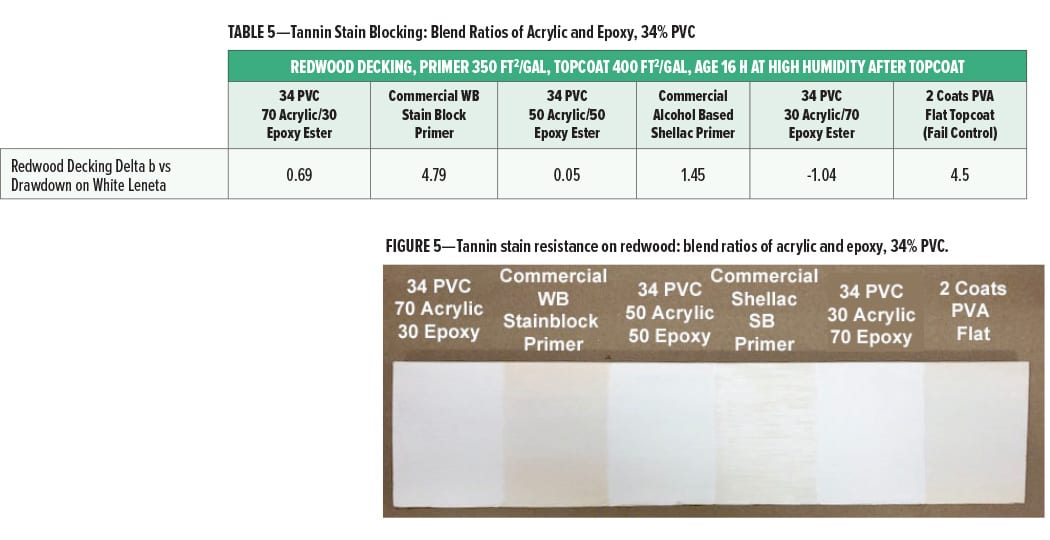
The tannin bleed resistance of the experimental primers was very good with Delta b values of less than 1.0, indicating little change in yellowness over the redwood panel compared to the control drawdown. At all blend ratios, the stain blocking was slightly better than the commercial shellac solvent-based primer and much better than the commercial water-based stain blocking primer. The slightly higher Delta b at a ratio of 70% acrylic and 30% epoxy ester compared to the 50% acrylic and 50% epoxy ester may indicate a slight drop off in tannin stain blocking at the 70/30 ratio, however. This may indicate that the maximum level of cationic acrylic that can be used in the primer to give optimal tannin stain blocking is about 70%.
Tannin blocking on redwood for different PVC (all at 50% acrylic and 50% epoxy ester ratio) is given in Figure 6 and Table 6.
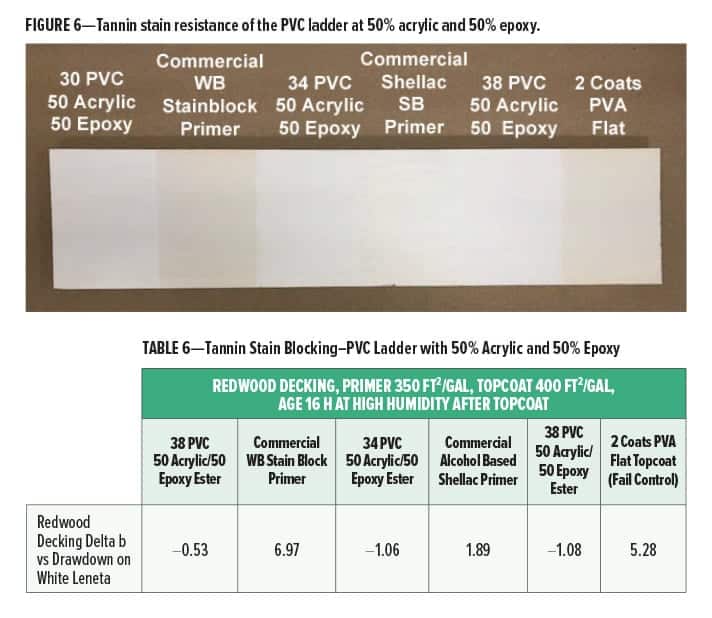
At a blend ratio of 50% acrylic and 50% epoxy ester, the tannin blocking was very good up to 38% PVC (Delta b values under 1.0). As in the polymer ratio study, the tannin block for all the experimental primers is better than the commercial water-based stain blocking primer and slightly better than the commercial shellac primer. The improved performance may be due to the lower pH of the cationic primers and to the cationic functionality on the polymer helping to tie up the phenolic tannins.
Sandability of the different primers is shown in Figure 7 and Table 7.
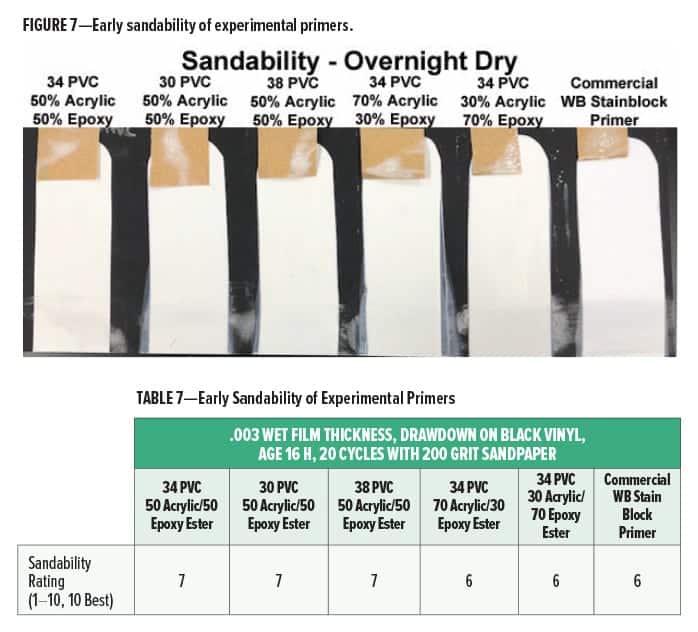
All primers had fair sandability after an overnight dry, with good dusting. However, there was some clogging of the sandpaper for the experimental cationic primers as well as the commercial water-based stain blocking primer. Contractors often prefer primers with good early sandability because they can sand a primed wall after an overnight dry before topcoating so this property may need to be improved. The early sandability must be balanced with good film flexibility, however. It is often difficult to formulate primers that have both early sandability and enough flexibility to resist cracking on wood when subjected to temperature and moisture changes.
The flexibility of two coats of the experimental primers was tested using thermal and moisture cycling on yellow pine. The results for five cycles of thermal and water soak cycling are given in Table 8. The experimental primers were compared to the commercial primers and a primer made with 100% of the epoxy ester.
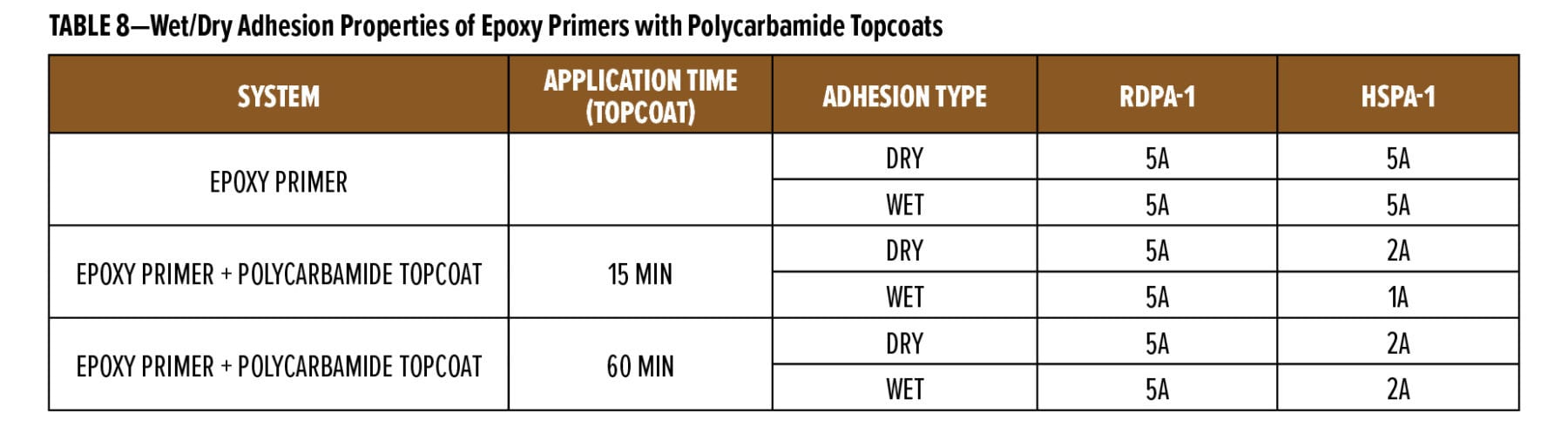
The different rates of thermal and moisture expansion of the early and late wood grain in the yellow pine stresses the paint film along the grain lines and gives an indication of the flexibility and crack resistance of the primer coat. The experimental primers had good crack resistance in this test and were better than the commercial water-based stain block primer. The blends of the epoxy ester with the acrylic were also better than the primer made with only the epoxy ester, showing that the cationic acrylic helps improve the flexibility of the epoxy ester. Exposure testing to verify the crack resistance of the experimental primers is in progress.
Wet adhesion (after one-hour water soak) of the midpoint experimental primer (34 PVC, 50% acrylic, 50% epoxy ester) and the commercial water-based stain blocking primer to gloss alkyd and metal substrates is given in Table 9.
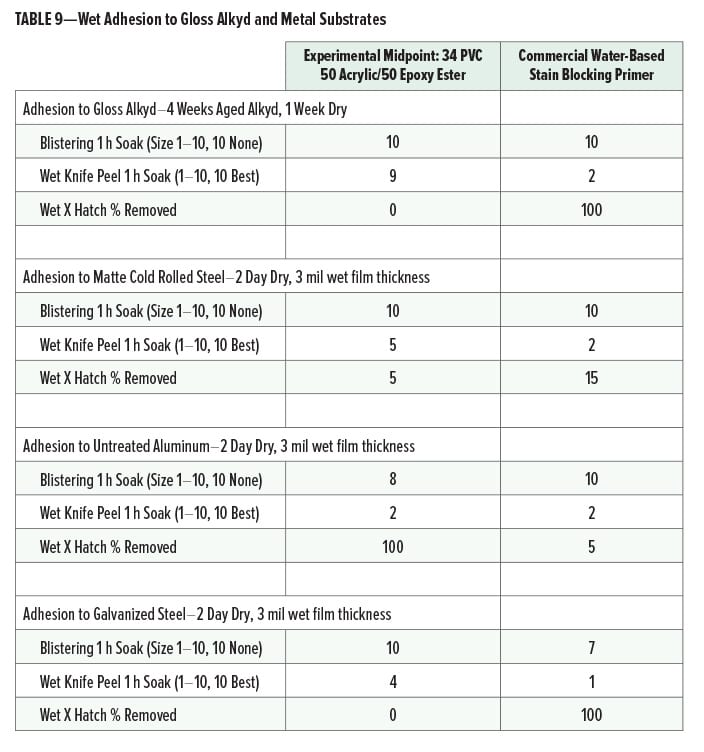
The experimental cationic primer had very good wet adhesion to aged gloss alkyd substrates. On metal, the experimental midpoint formula had good adhesion except on untreated aluminum. Adhesion of the commercial water-based stain blocking primer was poor on gloss alkyd and galvanized steel.
Conclusion
Blends of a cationic acrylic with cationic epoxy ester had very good tannin and washable marker stain blocking that was better than a commercial water-based stain blocking primer. The improved stain blocking may be the result of three factors: the acidic nature of the primer, the cationic reactive sites on the polymer, and the one coat stain blocking, which may result in better barrier properties. The experimental primers also had good crack resistance under thermal and moisture cycling on wood but only fair early sandability. Adhesion was also very good for the experimental primer (except on untreated aluminum). The overall performance was better than a water-based commercial stain blocking primer and was equal to or better than a commercial alcohol-based shellac primer. The blend of cationic epoxy ester and cationic acrylic has a balance of properties that make it suitable for potential use in a universal stain blocking primer.
References
- Burke, E., et al., “Understanding Extractive Bleed,” CoatingsTech, 3, 48–53 (2010).
2. Gruver, J. and Doll, P., “Waterborne Primers Providing the Base for Exceptional Topcoat Performance,” Paint & Coat. Ind., 10 (2018).
3. Stirling, R. and Morris, P.I., “Reducing Depletion of Western Red Cedar Extractives from Wood in Service,” U.S. Department of Agriculture, Forest Service, Tale of Two Cedars: International Symposium on Western Redcedar and Yellow-Cedar, 87–89 (2010).
4. Thomassen, I., “Stain-blocking and mildewcide resistant coating compositions,” US5460644A (1995).
5. Van Rheenen, P., “Coating composition containing ion exchange resins,” US6815466B2 (2003).
6. Klijn, T., Twene, D., and Mestach, D., “Stain blocking waterborne coating composition,” WO2005071023A1 (2005).
7. Walker, J. and Scott, C., “Tannin stain inhibiting coating composition,” US6245141B1 (2001).
8. Wildeson, J., Kelly, J., Cosyns, A., and Wiese, H., “Stain blocking compositions,” US20100124614A1 (2015).
9. Rokowski, J., Donnelly, K., and Kuhn, R., “Tannin stain blocking coated substrate,” US5527619A (1996).
10. Van Rheenen, P. and Chou, C., “Cationic latex coatings,” US5312863A (1994).
11. Feola, R. and Gobec, M., “New Waterborne Cationic Binders for Primer Applications,” Paint & Coat. Ind., 9, 86–90 (2005).
12. Paar, W., Feola, R., Gmoser, J. and Friedl, M., “Aqueous binders based on epoxy resins,” US6653370B2 (2003).
13. Hart, M.A., “Evaluation of Stain Blocking Primer Coatings with Low Volatile Organic Compound (VOC) Content,” California Polytechnic State University, digitalcommons.calpoly.edu/theses/482/ (2011).
14. Graystone, J.A., “Coatings for Buildings,” Paint & Surface Coatings—Theory and Practice, 2nd ed., 401 (1999).
15. Steiner, A., et al., “Epoxy Acrylic Hybrid Resins,” European Patent Application EP3381958A1 (2018).
16. Czora, Z., “Selecting Fillers for Membrane Coatings—How Do They Affect the Performance Characteristics of a Coating,” LinkedIn, www.linkedin.com/pulse/selecting-fillers-membrane-coatings-how-do-affect-czora-che-atsc (2017).
17. Hajas, J. and Du, J.W., “Stabilization of Titanium Dioxide in Non-Aqueous and Aqueous Coatings,” Paint & Coat. Ind., 6, 40 (2004).
18. ICL Specialty Products Incorporated,Halox Flash-X 330 Nitrite Free Liquid Flash Rust Additive (2018).
19. Specialty Polymers, Inc., RayCat 5428 Technical Bulletin (2018).
20. Allnex, Technical Data Sheet, Duroxyn EF2406 40WA (2014).
*Presented at the 2019 CoatingsTech Conference, April 8–10, in Cleveland OH.
CoatingsTech | Vol. 16, No. 5 | April 2019
