By Philip L. Simer, BruggemannChemical U.S., Inc., and Jessica Linker, L. Brüggemann GmbH & Co. KG
Chemically converting monomer via oxidizing and reducing agents (redox) in post-polymerization is a widely used technique; however, little time or effort is typically invested in optimizing this step. Through redox optimization, cost savings can be realized via shortened cycle times while also reducing residual free monomer to non-detectable levels.
While temperature and pH play a major role in the kinetics of redox chemistry, these are variables that often cannot be altered. However, improvements can be achieved regardless of these conditions as well as process or equipment restraints. A few of the variables that impact reaction time in the post-polymerization step that can more easily be optimized include redox pair, dosage/concentration, rate, and method of addition. A series of tests were performed to study the impact of these factors.
Methods
In each test series, a latex using persulfate to thermally initiate main polymerization was used. In all cases, the polymers were chased with redox expressed as a weight percentage of actives content on total latex. Tertiary-butyl hydroperoxide (tBHP) was used throughout all tests as the oxidizer.
In the first test series, the specifications of the latex were as follows: 41% non-volatiles, 6 °C Tg, pH 2.6, residual monomer before chase of ~30,000 ppm (predominantly BA). The monomer make-up consisted of methyl methacrylate (MMA), styrene (Sty), methacrylic acid (MAA), and butyl acrylate (BA).
Various redox dosages were tested in this series, as well as several reducing agents, including sodium metabisulfite (SMBS), sodium salt of sulfinic acid derivative (SSIA), sodium salt of sulfonic acid derivative (SSOA), and zinc salt of sulfonic acid derivative (ZSOA).
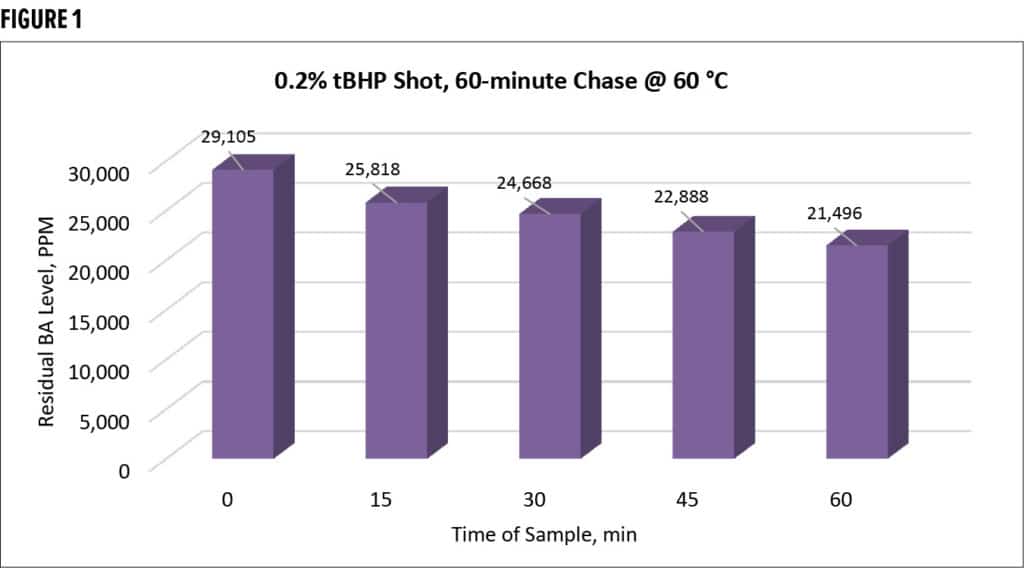 Different methods of addition were also tested, including simultaneous, continuous feeds at various times and one-shot additions. TBHP was also utilized without any reducing agent as a control, as seen in Figure 1. All testing was done at 60 °C and samples were pulled every 15 minutes to test for free monomer via Headspace GC/MS.
Different methods of addition were also tested, including simultaneous, continuous feeds at various times and one-shot additions. TBHP was also utilized without any reducing agent as a control, as seen in Figure 1. All testing was done at 60 °C and samples were pulled every 15 minutes to test for free monomer via Headspace GC/MS.
In the second test series, the specifications of the latex were as follows: pH 3.5, 40% non-volatiles and chase temperature 80 °C. The monomer make-up consisted of MMA, BA, and 2-ethylhexyl acrylate (2-EHA). The test series utilized tBHP at 0.02% with various reducing agents at 0.015%.
Reducing agents tested included: sodium formaldehyde sulfoxylate (SFS), SSIA, SSOA, modified sodium salt of sulfonic acid derivative (MSSOA), and reduced formaldehyde SFS (RFSFS). The redox pair was charged as one-shot of each component.
The third test series was conducted on a latex with the following specifications: pH 7.5, 50% non-volatiles and chase temperature 80 °C. The monomer make-up consisted of styrene (Sty), 2-ethylhexyl acrylate (2-EHA) and butyl acrylate (BA). Once again, several reducing agents were tested in this series, including SSIA, SSOA, MSSOA, and RFSFS. The redox pair was dosed at 0.1% of each component, however tBHP was charged as one-shot and the reducing agent was fed over 30 minutes thereafter.