By Dieter Groβschartner and Ingo Giesinger Sun Chemical, Nussdorf, Austria Jonathan Doll and Jason Eyink Sun Chemical, Cincinnati, United States
Liquid coatings and inks are facing increased pressure with respect to the presence of volatile organic compounds (VOCs). As a result, ink and coating formulators have to balance safety, regulatory, and emission standards while meeting their customers’ performance requirements. For low-VOC, metallic formulations, aluminum pigments are used to impart a desirable silver or metallic effect with excellent hiding power. In these applications, aluminum is typically supplied as either a dry powder or waterborne preparation, however, stringent safety measures are required to prevent dust explosions and corrosion, which can release hydrogen gas. In this article, a versatile, VOC-free aluminum preparation is introduced that can be formulated into a variety of solvent- and waterborne inks and coatings while directly addressing the aforementioned safety concerns. Minimum explosion energy (MIE) and gassing data, as well as formulation and performance data are presented for both water- and solvent-based systems.
Introduction
Aluminum pigments are a class of effect pigments that can impart a desirable silver to gray color with a pronounced metallic effect. Like most effect pigments, aluminum (or more generally metallic) pigments impart an appearance in inks and coatings that is due, in part, to the shape of the pigments. If one were to make a display using spherical aluminum powder, the end result would be dull, gray, and would have very little of the characteristic light-to-dark color travel known as the flop. As the shape of the aluminum particles changes from spherical to platelet-shaped, aluminum pigments begin to display a flop effect, and a bright silvery appearance characterized by high brightness (L*) values and gloss due to reflection of light off the platelet face or surface. Additionally, the platelet-shaped particles would show much better hiding power than their spherical counterparts. For these reasons, most, if not all, of the aluminum pigments currently available on the market are platelet-shaped.
To achieve the desirable platelet shape, aluminum pigments are traditionally produced via ball milling. Ball milling is a process whereby a horizontal mill is loaded with media and aluminum powder. As the mill rotates, the media impacts the aluminum powder (grit) and hammers it into a flake shape. Typical ball milling processes will have three main ingredients: aluminum grit, a hydrocarbon solvent, and a lubricant. The purpose of the hydrocarbon solvent and the lubricant is to minimize the amount of water in the mill, thereby reducing oxidation and decreasing the probability of cold welding and aggregation of the aluminum. Out of the mill, the final pigment will have appearance and properties that are determined by the mechanical and chemical inputs of the process.
Milled aluminum typically has two main shapes, known as corn flake and silver dollar, that are defined predominantly by the mechanical inputs of the milling. Corn flake pigments have an appearance that is similar to the breakfast cereal—a flat, smooth to bumpy platelet with ragged, irregular edges. Silver dollars, on the other hand, have a coin-like appearance, with rounded edges and a smooth surface. Of the two, silver dollars tend to have a more brilliant appearance with higher gloss and travel because their smooth surface minimizes the number of scattering centers in the pigment, while corn flakes are duller and whiter due to the greater degree of scattering.
The choice of lubricant also has an impact on the appearance and the wetting behavior of the pigments. Typically, the lubricants used are the saturated and unsaturated analogues of C-18 fatty acids, namely stearic and oleic acid, respectively. When stearic or other saturated fatty acids are used, the aluminum pigments will have a low surface energy that causes them to rise to the air-liquid interface of inks and coatings with a sufficient surface energy mismatch. The pigments crowd the surface and have superior alignment in the coating, resulting in a minimization of scattering centers and a high-gloss appearance. These types of pigments are known as leafing. When oleic or unsaturated fatty acids are used, the pigment surface is wetted by the coating and thus the pigments are evenly distributed through the thickness of the coating, giving a duller metallic appearance known as non-leafing behavior.
With the broad range of shapes and surface behavior, ink and coating formulators have a wide variety of metallic effects and appearances from which to choose. With this in mind, traditional aluminum pigments have some significant 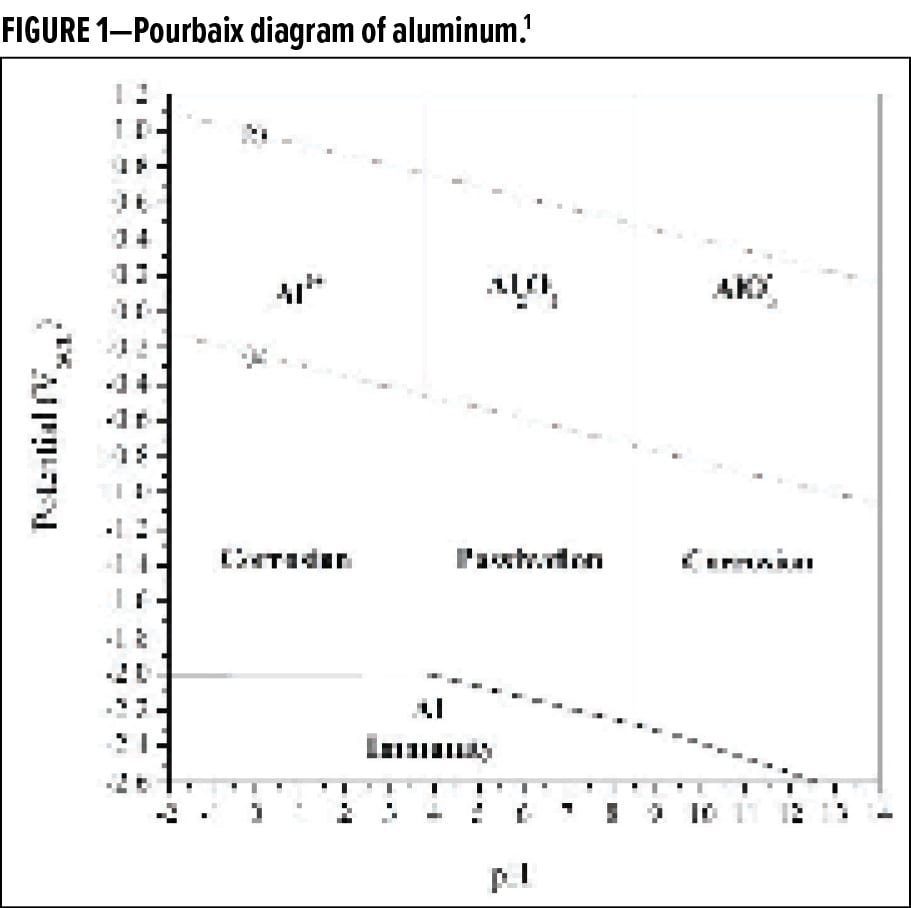 limitations that restrict their use to solventborne (SB) applications. The largest challenge when handling aluminum pigments is related to the fact that aluminum is a reactive metal and will readily react with water, acids, and bases, as illustrated by the Pourbaix diagram in Figure 1.1 The figure shows that aluminum at 0 V potential will react under all pH conditions in an aqueous setting, resulting in corrosion at acidic and basic pH and oxide growth at neutral pH. Due to size and surface area considerations, pigmentary aluminum will react much more quickly under aqueous conditions, causing the release of hydrogen gas according to equation (1).
limitations that restrict their use to solventborne (SB) applications. The largest challenge when handling aluminum pigments is related to the fact that aluminum is a reactive metal and will readily react with water, acids, and bases, as illustrated by the Pourbaix diagram in Figure 1.1 The figure shows that aluminum at 0 V potential will react under all pH conditions in an aqueous setting, resulting in corrosion at acidic and basic pH and oxide growth at neutral pH. Due to size and surface area considerations, pigmentary aluminum will react much more quickly under aqueous conditions, causing the release of hydrogen gas according to equation (1).
Al + 3 H2O Al(OH)3 + 1½ H2 (1)
In waterborne (WB) formulations containing aluminum, the release of hydrogen gas can cause packaging to bulge, and, in extreme cases, to burst, leading to product loss and large messes. In minor cases, it can cause the release of foam or lead to color changes in the product. To mitigate these issues, aluminum pigment manufacturers have developed two classes of treatments: 1) the creation of a physical barrier around the aluminum pigment; and 2) chemically modifying the surface of the pigment.
In the first strategy, a highly crosslinked layer is deposited on the aluminum pigment. This layer may be inorganic (i.e., SiO2) or organic (i.e., polymer). Generally, this strategy produces the best protection from water; however, products treated this way are expensive. Thus, it is a strategy used only for higher-end inks and coatings that can tolerate the cost. They are commonly used to meet the demanding specifications of automotive coatings. The second strategy uses small molecules such as phosphates, chromates, molybdates, etc., to passivate the surface and form an impermeable barrier. This strategy is less expensive, although these layers may be disrupted by drastic pH changes or concentration gradients. In some cases, hydrophobic treatments can affect compatibility in some WB systems. Whatever strategy is used, both methods provide protection of the aluminum pigments from attack by moisture under ambient conditions.
Another drawback of aluminum pigments is related to the form in which they are supplied. Typically, aluminum pigments are supplied as a paste with a hydrocarbon solvent like mineral spirits. This can lead to issues with solvent compatibility in inks and coatings, and also make the aluminum pigments a source of volatile organic compounds (VOCs), which have come under increased regulatory scrutiny across the inks and coating industries. While other solvents can be used to enhance solvent compatibility, these other solvents usually do not address the issue of VOCs in the formulations. If VOCs are a concern, formulators are often forced to choose between aluminum pigment powder, which is potentially explosive and can be dangerous to handle in large quantities, or an aluminum pigment preparation.
Aluminum pigment preparations take many forms, including both wet and dry compositions. Pellets are typically the most common form for dry formulations. Typical pellets are comprised of a resin or other substance that is combined with aluminum pigments and dried. Loading of the aluminum in pellets can be low, and coatings and ink formulators have to accommodate the extra ingredients in the pellets. In addition, pellets may only have compatibility with SB or WB formulations.
In this article, a new high pigment content aluminum preparation that can be formulated into both WB and SB inks and coatings is introduced. The pigments in these preparations are chemically passivated to protect against moisture-triggered corrosion. Additionally, the preparation is in the form of dry pellets, making it a dust-free, ultra-low VOC product. The pellets are composed of 90% aluminum, allowing for a high degree of formulation flexibility. Moreover, any type of aluminum can be used in the pellet, from fine leafing corn flakes to large, non-leafing silver dollars. The properties with respect to appearance and stability in WB and SB inks and coatings are presented and discussed.
Materials and Methods
Pellets Tested
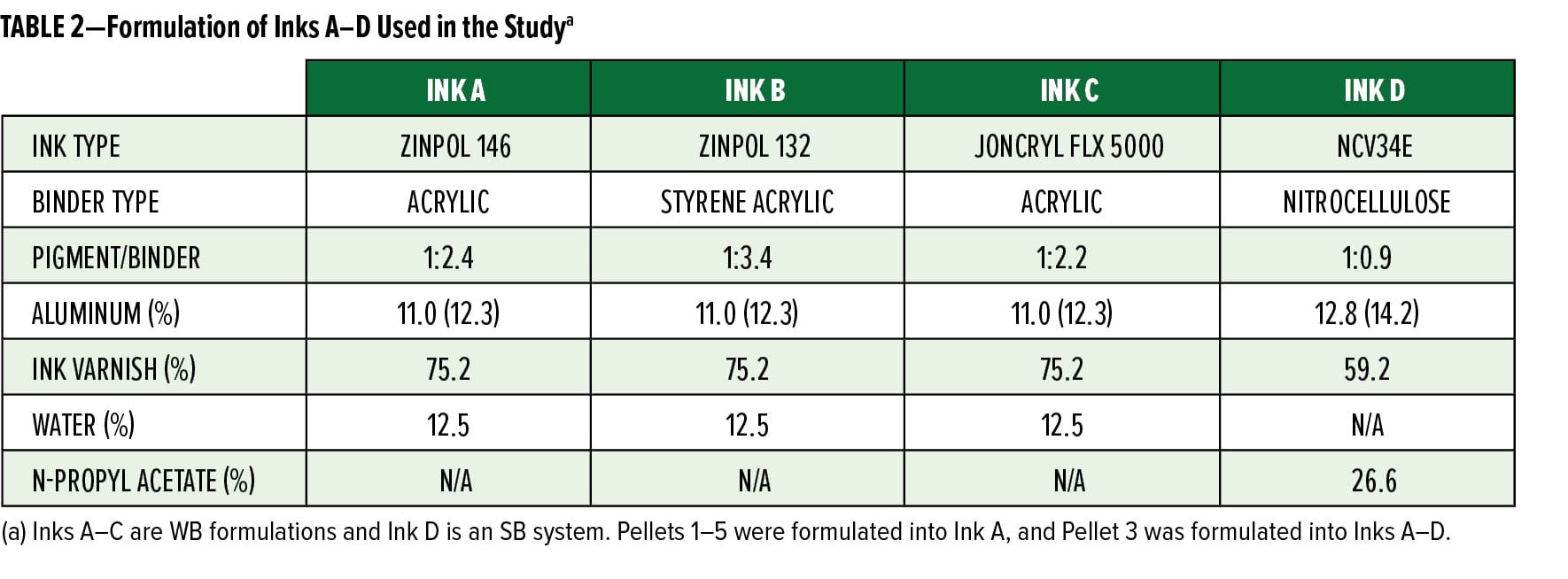
Five different aluminum preparations were made and tested for color and dispersibility in a WB ink. Table 1 lists the pigments tested and includes information about the flake type and particle size. For simplicity, Pellet 3 is used as the main preparation for most of the study, unless indicated otherwise.
Incorporation into WB and SB Inks
To assess the color and appearance of the preparations in ink applications, Pellets 1–5 were dispersed into a WB acrylic ink system (Ink A). Additional measurements of color and dispersibility were done by combining Pellet 3 with two additional WB inks (Ink B and C) and one SB ink (Ink D). The performance of Pellet 3 in Inks A–D was compared to Maxal 64064, a non-leafing silver dollar flake of similar particle size distribution. Some general formulation information for inks A–D is shown in Table 2.
To incorporate the preparations into the WB formulations, the pellets were soaked in an equivalent weight of water for 15 min. After the soaking period, the pigments were combined with the varnish and any additional ingredients and mixed with a high-speed disperser for about 30 min or until the pellets were fully dissolved. The inks were drawn down on a black and white display paper using a #2 k-bar and allowed to air dry.
To incorporate the preparations into the SB formulations, the pellets were soaked in an equivalent amount of isopropyl acetate for 60 min. After the soaking period, the pigments were combined with the varnish and any additional ingredients and mixed until the pellets were fully dissolved. The ink was drawn down on a black and white display paper using a #2 k-bar and allowed to air dry.
Incorporation into WB and SB Coatings
To assess the color and appearance of the preparations in coatings applications, Pellet 4 was separately dispersed into a WB epoxy coating and a WB acrylic system and compared to a powdered aluminum of similar particle size, Benda Lutz 2051. Some general formulation information for the coatings is shown in Table 3.
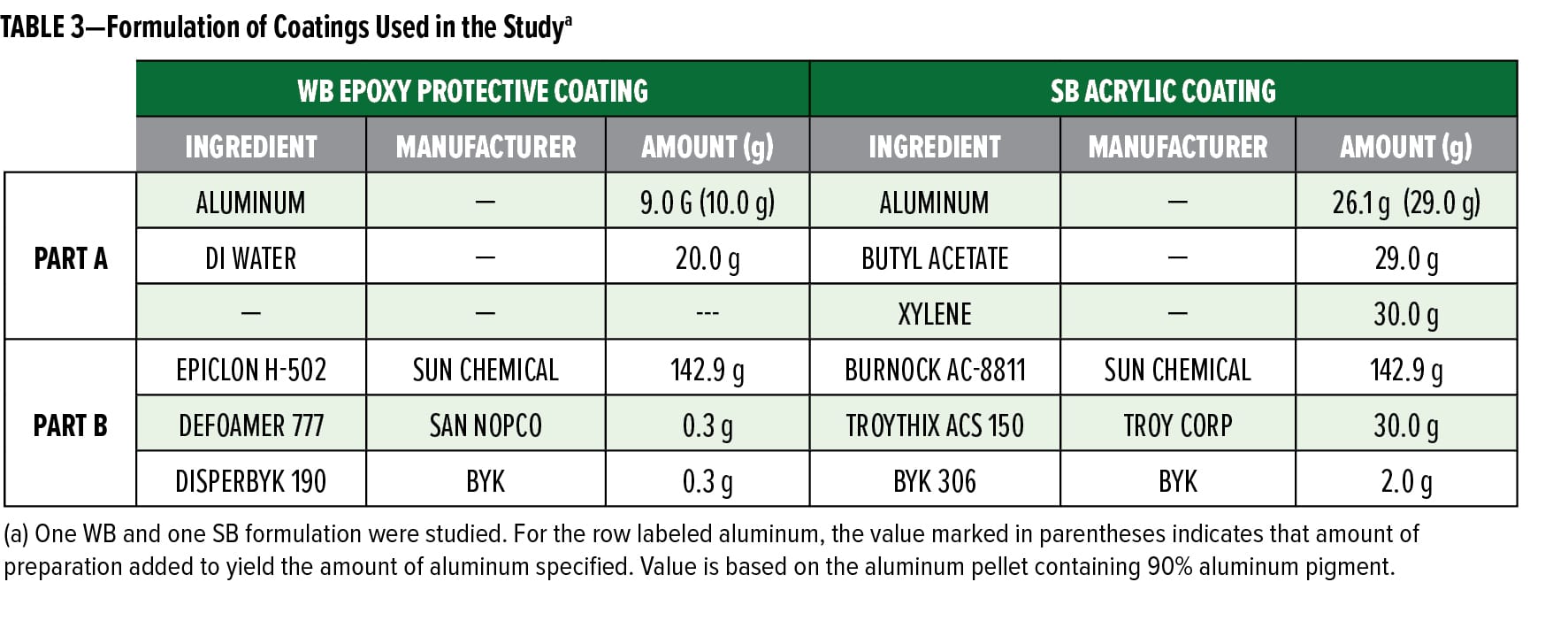
For each coating, the preparation was incorporated into the premix formulation described in Table 3, Part A. Once combined, the components of Part A were soaked for 30 min followed by mixing for 30 min. While Part A was being prepared, the components of Part B were being combined. Part A was gradually added to Part B, and the entire mixture was stirred for 30 min. The coatings were drawn down using a 3 mil (75 micron) wet Byrd applicator on black and white coated Leneta paper, and their appearance was analyzed.
Appearance Testing of Samples
For each of the samples, a few parameters were measured: gloss at 60° (G60), L* with a 45° incident beam and 15° aspecular reflection angle (L15), and the flop index (FI). The gloss is a measure of the specular reflection of the display. The L15 is a measure of the color or brightness of the pigment, and the FI is a measure of the color travel observed at different aspecular viewing angles. The FI is defined by equation (2), where L110 is identified as L* with a 45° incident beam and 110° aspecular reflection angle, and L45 is identified as L* with a 45° incident beam and 45° aspecular reflection angle.
![]() (2)
(2)
The gloss measurements were performed using a BYK Micro Tri Gloss glossmeter operating at 60° incident angle, and the L values were measured using an X-Rite MA-98 multiangle spectrophotometer using D65 light.
Gassing Stability Test
To test the gassing stability in pure water, 20 g of aluminum (adjusted for aluminum content—22.2 g preparation) was added to a 250 mL Erlenmeyer flask equipped with a stir bar. The preparation was compared to an unpassivated aluminum powder, Benda-Lutz 2051 (Sun Chemical), that has a similar particle size distribution to Pellet 4. Next, 50 mL butyl glycol and 50 mL deionized water were added to the flask. The flask was sealed and mixed until all pigment was well dispersed. Following mixing, the flask was added to a 40°C water bath, connected to a gas burette, and stirred for 30 days. The evolution of hydrogen gas was monitored by the displacement of water in the gas burette. The test was stopped and considered a failure if over 120 mL of gas had been generated. This test was performed for both the 2051 pigment and Pellet 4.
In separate tests, the test was modified so that Inks A–C were added to the Erlenmeyer flask at the beginning. The inks were modified so that the total amount of aluminum, water, and varnish in the system was 18.2%, 11.7%, and 70.1%, respectively, and the total aluminum content was equal to 20 g. This was to measure the gas evolution in the finished ink systems.
Storage Stability
To assess the storage stability of the preparations, ~10 g of preparation was hot sealed in a bag under ambient conditions. The samples were stored for up to a year at room temperature. The dispersibility and color of the samples were monitored over this time.
Results and Discussion
General Description of Pellets
The aluminum pigment preparation is a pelletized material with a diameter of ~2 mm. They are composed of 90% aluminum pigment and are designed to formulate into WB and SB formulations. There are five different grades based on aluminum pigment shape and leafing behavior. In this article, Pellets 3 and 4 are described in most of the analyses for the sake of brevity, but similar trends hold for all of the grades. The pellets are completely dry and contain little to no VOCs. In addition, the size of the pellets makes the measurement of the minimum ignition energy (MIE) impossible as the preparation is too large to ignite as a dust.
Appearance in Ink Formulations
To assess the appearance and to determine if they were similar in dispersion behavior, the five pellets were separately dispersed in a WB acrylic ink and drawn down. The relevant coloristic parameters were measured and are compared in Table 4. Each of the different pellets has an appearance that is unique with respect to the others. The pellets show similar behavior as to what would be observed in standard milled flakes with silver dollars showing higher brightness, gloss, and flop than the corn flakes. Within the corn flake materials, the leafing grades shows higher gloss.
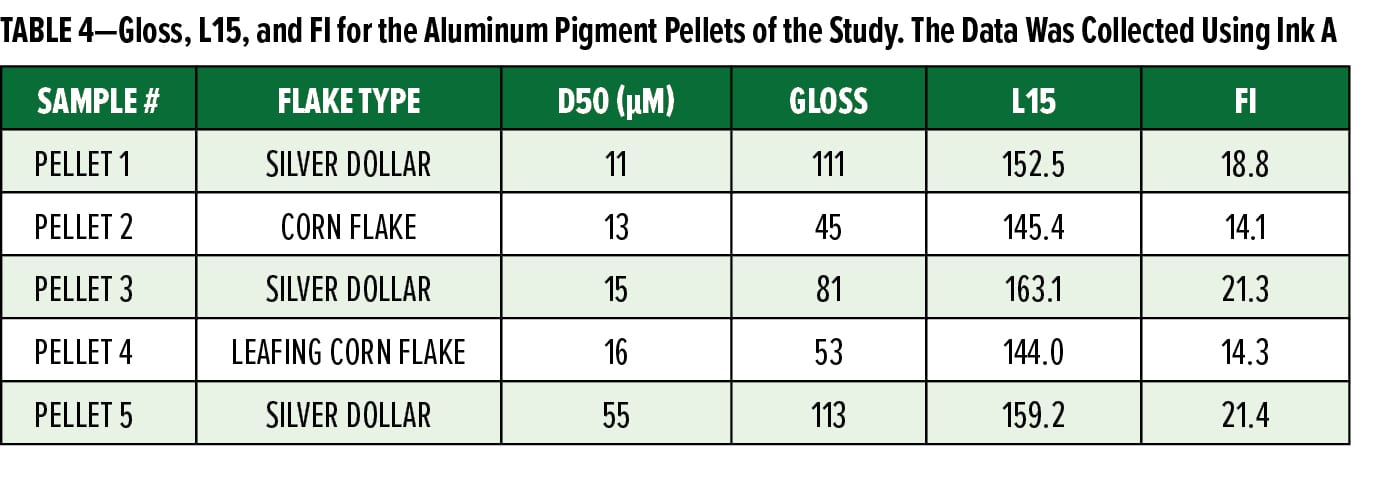
To better understand how the pellets perform in different ink formulations, Pellet 3 was compared to Maxal 64064 (Sun Chemical) in three different WB ink formulations (Inks A–C) and one SB nitrocellulose ink formulation (Ink D). The inks were mixed, drawn down, and the coloristic properties were measured and are reported in Figure 2. In every ink, Pellet 3 had similar performance to the Maxal 64064 with respect to appearance, although the degree of difference between the two pigments was system dependent. It is also worth noting that the Maxal 64064 required soaking in a cosolvent to be fully dispersed in the WB ink system without aggregation, while Pellet 3 only required a soak in water. This data shows the compatibility of the aluminum preparation in both WB and SB inks and indicates at the overall formulation flexibility of the system.
Ink Formulation and Stability
For WB inks, such as Inks A–C, one of the most important criteria is the stability to corrosion and gassing. Corrosion leading to the release of hydrogen gas is one of the largest failure modes of WB systems, and can cause issues ranging from discoloration to package bulging and potentially explosions if the packaging fails catastrophically. To assess the stability of the WB metallic formulations, a number of tests have been developed that study the stability of aluminum pigments in water. One of the most common tests performed involves dispersing the aluminum pigments in a mixture of butyl glycol and water and holding at constant temperature while the gas evolution is measured via water displacement in a gas burette over a period of 30 days. The failure criteria vary from test to test; however, it is generally accepted that a product clearly fails if the complete volume of water is displaced over the testing period.
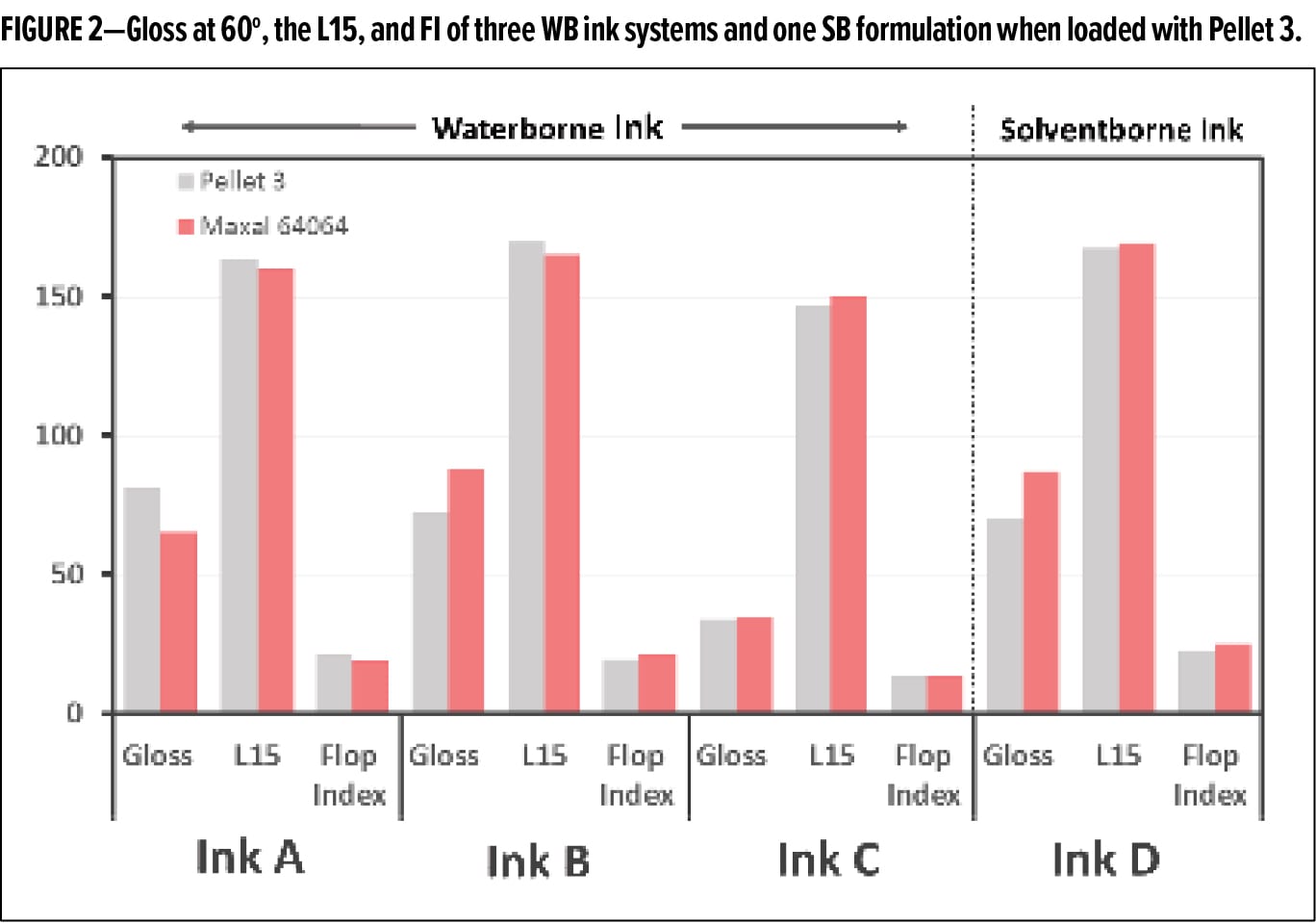
Figure 3a shows the results of the standard gassing test for a standard aluminum pigment powder, Benda-Lutz 2051, vs Pellet 4, which is an aluminum preparation containing pigment of similar particle size distribution. While the unpassivated aluminum pigment generated a considerable amount of gas, exceeding the measurement capabilities of the test, Pellet 4 remained stable under the 30-day test period, suggesting that it is well passivated towards water. In reality, this test only shows the stability of the pellet formulation to residual humidity or moisture in an accelerated environment and does not represent what will happen once the pigment preparation is added to an actual WB formulation. To quantify the behavior of aluminum pigments in application systems, the gassing test must be modified (see below).
Many WB inks and coatings contain ingredients that can accelerate the corrosion of aluminum and thus the susceptibility to gas generation. The oxidation may be due to interactions of the aluminum with the various pH adjusting chemicals that are used in inks and coating systems, such Lewis acids (phosphates) or Lewis bases (amines). Beyond causing localized pH swings, these types of molecules have the potential to catalyze oxidation even at neutral pH, or can interact with the chemical passivant to produce an unpassivated aluminum.
To test the aluminum pigment preparation’s performance, it was dispersed in Inks A–C, and subjected to annealing at 40°C for 30 days. The results are reported in Figure 3b. In general, none of the inks showed a level of gassing that would be considered problematic, with only 5 mL of gas being generated in Ink C. From these data, it can be concluded that the pellet is stable in these three formulations as well as in water. Moreover, this data suggests that the behavior of the pellets in other WB systems will be similar and that the aluminum preparation is stable in under aqueous conditions.
Appearance in Coatings Formulations
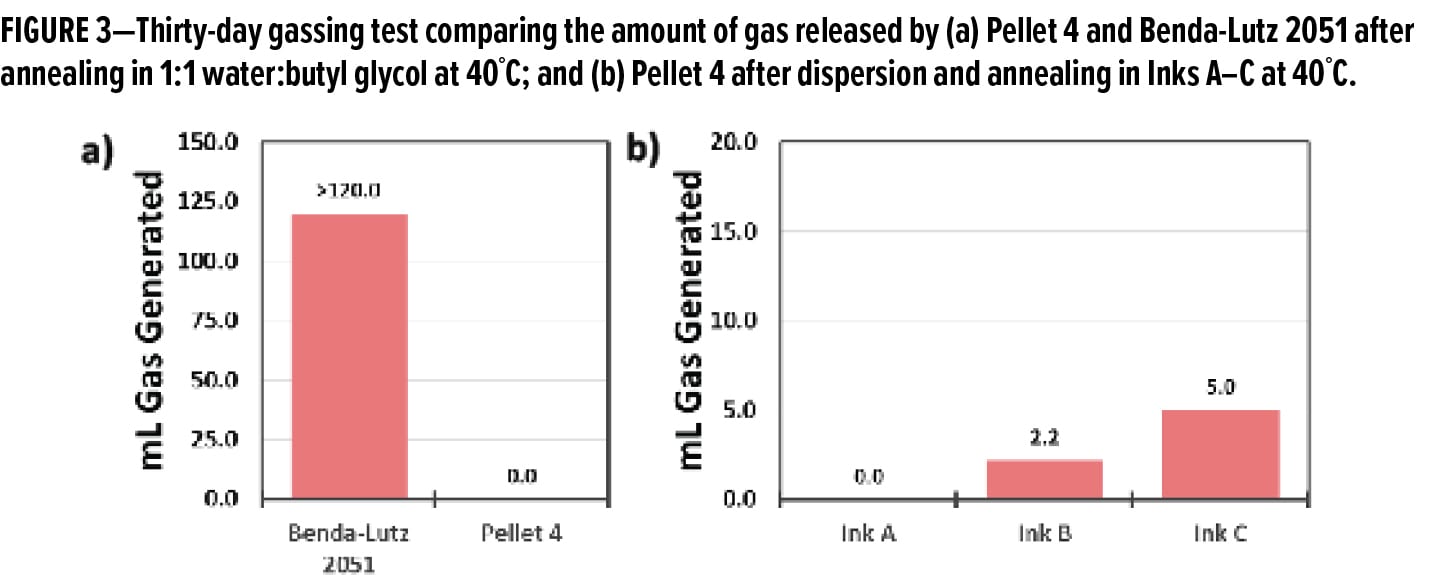
In addition to inks, the pelletized aluminum preparation can be formulated into both WB and SB coatings systems. In Figures 4a and b, the appearance of Pellet 4 in a WB epoxy protective coating and an SB acrylic coating, respectively, is compared against Benda-Lutz 1051, which has a similar particle size distribution and morphology to the aluminum used in Pellet 4. In Figure 4a, for the WB epoxy coating, it is seen that Pellet 4 outperforms 1051 in terms of gloss, brightness, and flop. This suggests that the pellet has better orientation and lay in WB, reflecting its better compatibility in this system.
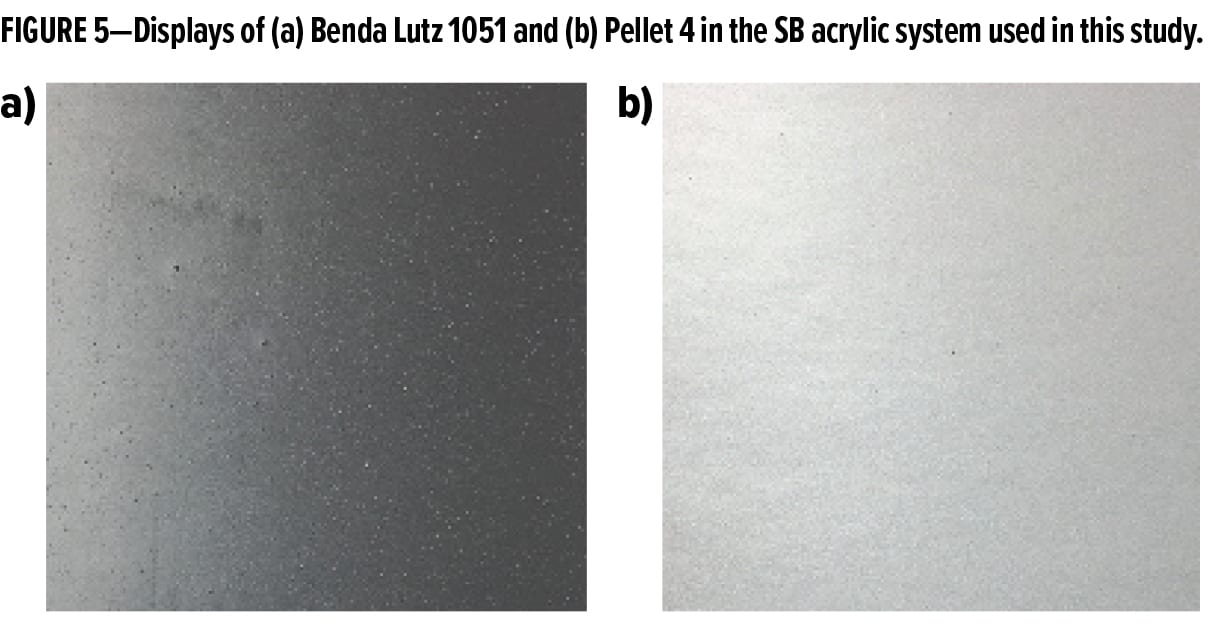
In the SB acrylic system (Figure 4b), Pellet 4 is slightly lower in L15 and flop compared to the Benda-Lutz 1051 pigment, suggesting that the orientation is not as good in this system. Benda Lutz 1051 is much higher with respect to gloss; however, it has a stronger leafing behavior than Pellet 4 in this system.
This result is slightly misleading however, and visual evidence in Figure 5a shows that the Benda Lutz 1051 is not totally dispersed in the coating, while Pellet 4 (Figure 5b) has uniformly dispersed without aggregates. This suggests that the component in the pellet composition helps with dispersion, not only in water, but also in SB systems.
Storage Stability of Aluminum Preparation
The storage stability of all of the pellets was assessed over a one-year period. Typically, aluminum pigments will begin to agglomerate and shift in color with exposure to air and humidity. Because of this, the typical shelf life of uncoated aluminum pastes and powders is generally accepted as six months. To assess the storage stability, the pellets were placed in a sealed bag for one year under ambient conditions, and the color and particle size distribution were measured. Ink A was used as the application medium. If there is a change in the color or particle size, it indicated that oxidation has happened, resulting in discoloration and/or agglomeration.
The results of the storage stability test are reported in Figure 6 for Pellet 4. This test shows that there is little change in the aluminum pigments during the storage period, and that the pellet shows stability for up to a year.
Conclusions
A series of pelletized aluminum preparations have been developed that can be formulated into both waterborne and solventborne ink and coating formulations. The pellet form is dry and large, containing minimal VOCs, and reducing the probability of dust explosions. The high aluminum content gives formulators broad flexibility when adding to their systems, while the stability and dispersibility in both water- and solventborne systems allows for the use of only one ingredient across formulations, simplifying inventories. Finally, the preparation has a long storage stability, and can be taken off the shelf for up to one year.
References
- Sukiman, N.L., Zhou, X., Birbilis, N., Hughes, A.E., Mol, J.M.C., Garcia, S.J., Zhou, X., and Thompson, G.E., “Durability and Corrosion of Aluminium and Its Alloys: Overview, Property Space, Techniques and Developments, Aluminium Alloys—New Trends in Fabrication and Applications,” Ahmad, Z. (Ed.), InTech, (2012). DOI: 10.5772/53752. Available from: https://www.intechopen.com/books/aluminium-alloys-new-trends-in-fabrication-and-applications/durability-and-corrosion-of-aluminium-and-its-alloys-over
CoatingsTech | Vol. 15, No. 9 | September 2018