By Monica Harvey, Natnael Behabtu, and Christian Lenges, DuPont Industrial BioSciences and Richard Milic and Jarmila Vlasakova, Synpo
Enzymatic polymerization is emerging as a scalable process to polymerize sucrose into engineered polysaccharides. Polymer architecture and material properties can be controlled selectively to provide the foundation for novel differentiated biomaterial platforms. One first example for such an engineered polysaccharide is alpha-1,3-polyglucose (alpha-1,3-glucan), which was prepared using this bioprocess. This article describes initial results applying this polymer in architectural coating applications. With the ability to control polysaccharide particle morphology, crystallinity, as well as overall polymer functionality and particle size, it was envisioned that polysaccharides can be designed to provide performance advantages for paint applications.
Introduction
Advances in new material technology to further improve the performance of formulated products across various end-use markets continue to drive innovation and economic growth. At the same time, it is becoming increasingly paramount that these materials are also sourced from sustainable, often renewable feedstocks ideally through benign processes within a circular economy framework.1
Progress in the development of architectural and industrial coatings has focused on providing improved paint performance while also optimizing aspects such as pigment efficiency (e.g., reduction of TiO2), moisture management and transport, and gloss reduction while maintaining key properties such as abrasion performance and overall mechanical properties. At the same time, continued emphasis has been placed on reducing the environmental footprint through lower volatile organic content (VOC) in paint systems. In addition, increasing efforts have been directed towards introducing more sustainable material alternatives and eventually replacing typical building blocks in coatings formulations with them.2
However, it is still challenging to identify performance advantaged renewable building blocks that are accessible at an affordable cost, and are also based on fungible, readily available raw materials produced in a sustainable and scalable industrial process. DuPont continues strategic material development programs to achieve these objectives.
This article describes details of the use of engineered polysaccharide materials derived from the polymerization of sucrose, specifically alpha-1,3-polyglucose (alpha-1,3-glucan) in coating applications. The general material properties and the use of this material in combination with solventborne alkyd paint systems are described, and various coating properties are discussed.
In particular, one coating property of relevance for the application of alpha-1,3-glucan is its impact on gloss reduction. Initial results showing the utility of this material as a matting additive in alkyd coating systems are introduced.
In many coating applications, a matte surface finish is preferred. The gloss of a coating depends on the pigment volume concentration (PVC) of the paint, which can often be adjusted during production by the addition of suitable pigment extenders like talc. These often influence mechanical properties, optical properties, and the production process for alkyd and acrylic paint formulations. A preferred way is the specific addition of matting agents, typically selected from a range of fumed or precipitated silica, polymeric matting agents, or other compounds with high surface area.3 The matting agent must be inert, stable under environmental application and use conditions, and must be easily dispersible in most typical coating systems. Ideally, these additives should also allow for the simple adjustment of the desired degree of matting with high matting efficiency at small addition levels without negative influence on the desired physical properties of the coated surfaces. High transparency of the coating is also beneficial.4,5
Fundamental Material Properties of Engineered Polysaccharide
Structurally, polysaccharides are highly diverse biopolymers composed of repeating glucose units linked via glycosidic bonds. Each repeat unit in the polymer chain has three potentially reactive hydroxyl groups—in most cases, one primary and two secondary hydroxyl groups. The polymer’s structural characteristics, such as linkage isomers, degree of polymerization, branching and material aggregation, and morphology, allow for these materials to be found in nature as structure forming, essentially insoluble highly aggregated materials (e.g., cellulose) or as water-soluble, thickening materials with often various biological functions (e.g., starch, various gums).
The interaction of individual polysaccharide polymer chains through hydrogen bonding into associated super-molecular structures allows for deliberate material engineering from the nano to micron scale. Increasing efforts are, for example, reported on various process options to access and control nano scale cellulose-based materials.6 Enzymatic polymerization allows for a novel, controlled path towards the engineering of nano to micron scale structures within aggregated polysaccharide materials.
Traditionally, polymer structural design has been achieved through the specific control of the polymer architecture during the polymerization processes (e.g., polyolefins, polyacrylics, etc.), either through the use of designed polymerization catalysts and/or the adjustment of polymerization process conditions.
The translation of this concept to the design of polysaccharide material is now under development. Efficient and scalable methods to apply a monomer (e.g., sucrose) based, controlled polymerization protocol using an enzyme catalyst to engineer polysaccharide type polymers have not been developed for commercial, large scale material applications.
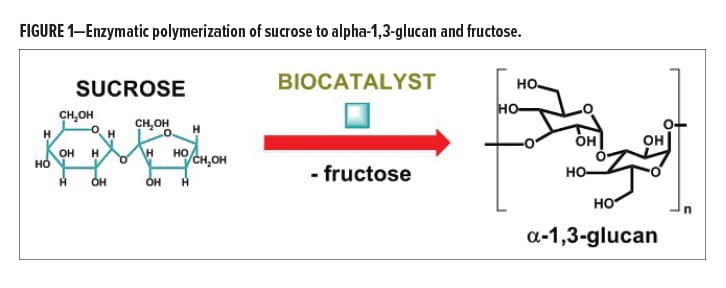
This article introduces a first example of this novel platform technology, with the specific enzymatic polymerization of sucrose to alpha-1,3-polyglucose (or short alpha-1,3-glucan) and fructose, as demonstrated in Figure 1. The polymerization process is carried out with the use of an enzyme catalyst to generate exclusively the linear polymer, which is water insoluble.
The specific family of biocatalysts is selected from the general class of glucosyltransferase (GTF) enzymes, and a typical process to produce these biocatalysts has been described.7 This polymer can be produced by contacting an aqueous solution of sucrose with a glucosyltransferase enzyme isolated from Streptococcus salivarius. For the work described here, the alpha-1,3-glucan utilized has a typical degree of polymerization of 800 glucose repeat units with a polydispersity in the range of 1.7–2.0, as controlled by the polymerization process conditions.6,8,9
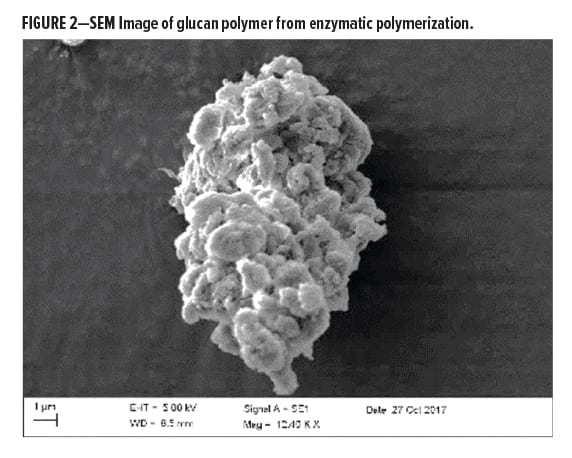
During the enzymatic polymerization process, the generated alpha-1,3-glucan polymer chains will associate through hydrogen bonding based on the reaction conditions and form primary particles with a particle size range between 10 and 30 nanometers. These will further aggregate and agglomerate during processing to form spherical particles in the range of about 1000–5000 nanometers (see Figure 2).
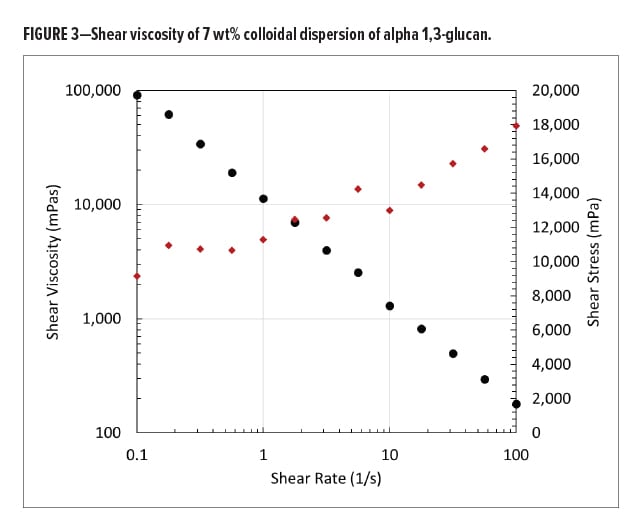
The polysaccharide isolated from this biopolymerization process is a free-flowing, white powder with high surface area.10 Although the alpha 1,3-glucan polymer is insoluble in water,11 it is hydrophilic with an open microstructure, and can be re-dispersed under shear to form a colloidal dispersion in water. These colloidal dispersions have high viscosity and are shear thinning.
Figure 3 shows the shear viscosity of a 7 wt% colloidal dispersion of alpha 1,3-glucan in water. The graph shows the typical shear thinning behavior of the material (black dots) and the shear stress of the sample (red dots). At low shear rate, the shear stress shows a plateau, indicative of the yield stress required for the sample to flow.
Figure 4 shows the viscosity of the colloidal dispersion increasing with solids loading. For concentrations >10 wt%, the system transitions from a flowing system to a soft solid.
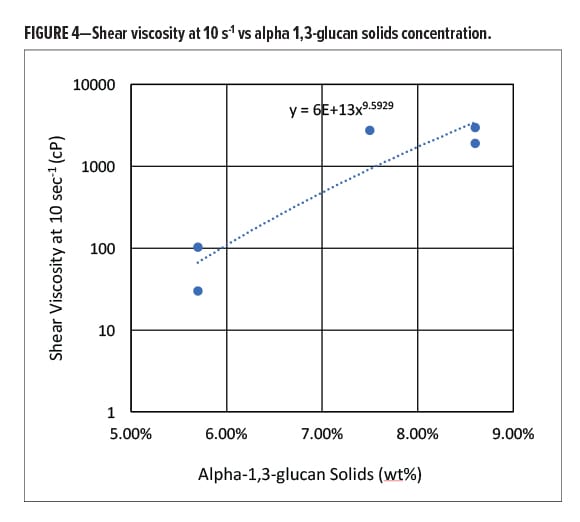
The transition from a flowable dispersion to a soft solid at low loading levels indicates that the spherical particles generate a highly structured phase.
This alpha-1,3-glucan material produced through enzymatic polymerization is of high compositional consistency and purity.12 The polysaccharide does not contain significant amounts of coproducts typically found in other polysaccharide materials used for industrial applications (e.g., lignin or hemicellulose in cellulose products or various proteins in starch products), the alpha 1,3-glucan isolated in this process typically contains < 0.2 wt% monosaccharide impurities. Based on the design of the enzyme, the process provides the target polymer with essentially exclusive selectivity, producing a linear polymer with greater than 99% alpha-1,3 linkages.13
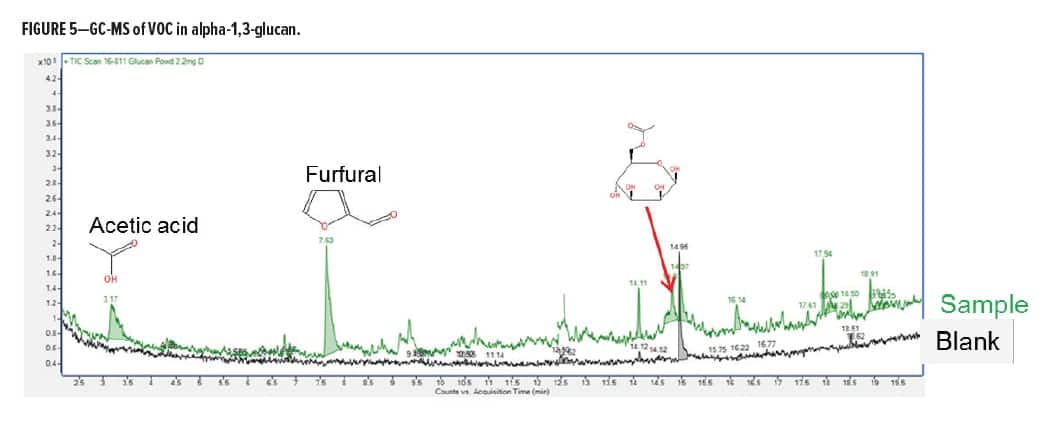
The isolated alpha 1,3-glucan polysaccharide was also analyzed by GC-MS after a typical thermal treatment cycle from 20 to 320ºC to identify VOC. The compounds detected at low levels were typical polysaccharide degradation products, such as acetic acid and furfural (see Figure 5); there were no aldehydes, NOx, SOx, or other volatiles detected. This supports the notion that the use of this type of material as a formulation component will advance the goal for increased sustainable material content in typical coating formulations.
Compatibility with Paint Systems
Based on the described characteristic of the alpha-1,3-glucan material to form stable colloidal dispersions in aqueous systems, the material can also be formulated directly into various water-based latex and solvent-based resin systems typically used in the coatings industry. For example, acrylic latex, ethylene vinyl acetate (eva) latex, alkyd resins, and epoxy resins all have been used in combination with this material to generate formulated systems.
High shear mixing is applied to effectively disperse the agglomerates of alpha-1,3-glucan into the latex or resin systems, which generates well dispersed formulations in which the polysaccharide is stable with on average micron or sub-micron-sized particles. These latex or resin dispersions are homogeneous and do not phase separate providing systems with excellent stability for typical applications of either water-based latex formulations or solvent-based resin formulations for paints and coatings.
As is common for the coatings industry, a pigment grind mill can be used for standard paint formulations and is suitable to achieve a good dispersion of alpha-1,3-glucan in many resin systems. The addition of dispersants or surfactants during the grinding may aid in achieving the desired degree of dispersion and the associated desired particle size and stability.
The ideal dispersant or surfactant is dependent on the resin system. For the solventborne alkyd resin system discussed here and in the following examples, typical dispersants are selected from the group of fluorosurfactants and siloxane-based dispersants, while in typical waterborne resin systems, such as ethylene vinyl acetate and acrylic latex systems, a typical dispersant may be based on water-compatible systems [e.g., 2-amino-2-methyl-1-propanol (AMP-95)].
Ease of processing in industry typical application equipment and infrastructure is important for the utility and commercial viability of new materials in coating applications. This new polysaccharide provides processing advantages for typical paint systems supporting the flexible use of this material across a range of typical formulations. The free-flowing powder is easy to dose into the formulation using standard equipment and does not show dusting issues common to many other micron-sized additives typically used in the industry, such as silicas or talc.
Experimental Section and Coating Results: Formulation Examples in Alkyd Paints
To illustrate the utility of the alpha-1,3-glucan material in a typical paint application, examples in combination with typical alkyd paint systems are described below.
The alpha-1,3-glucan is compatible with both waterborne and solventborne paint systems. The application example described details the formulation of alpha-1,3-glucan into a solventborne alkyd paint formulation and the performance of these coating formulations with regard to optical and mechanical properties.
Alkyd Resins: The formulation used for the solventborne alkyd paint base is shown in Table 1. A typical industry alkyd resin was used for the example formulations—a solventborne white paint based on soybean long-oil alkyd, with 62% oil length. This formulated alkyd paint base has a viscosity of 855 mPa.s (Brookfield, SCH 27/100, 23ºC), a total solids load of 45.4 wt%, and a PVC of 13.3%. In this formulation, a polymeric emulsifier was added to allow for the incorporation of water instead of an organic solvent to reach lower VOC.
To assess the performance of the alpha-1,3-glucan in this alkyd paint system, a pre-dispersion in water was generated. A 10% dispersion of the alpha-1,3-glucan was prepared in water under high shear, using 5 wt% (on dry alpha-1,3-glucan polymer) of a siloxane-based dispersant (Dynol 960). This dispersion was then blended using simple mixing equipment into the alkyd solventborne base paint at different concentration levels.
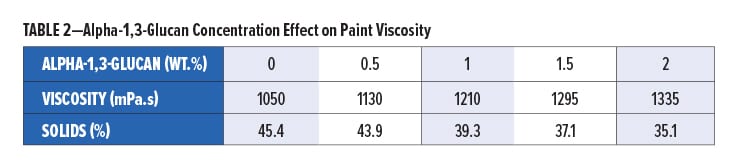
The effect of the addition of alpha-1,3-glucan on the viscosity of the paint formulation was measured, and the data is shown in Table 2. The polysaccharide imparts rheology modification and increases the viscosity of the overall formulation already at even low loading levels. The rheology imparted by alpha-1,3-glucan also reduces sag and splatter in typical draw-downs while helping to stabilize the pigment load.
Films of each formulation were drawn down using a rod applicator to yield samples with a dry film thickness of about 40 µm. The films were conditioned at 23ºC and 50% relative humidity before testing for optical and mechanical properties.
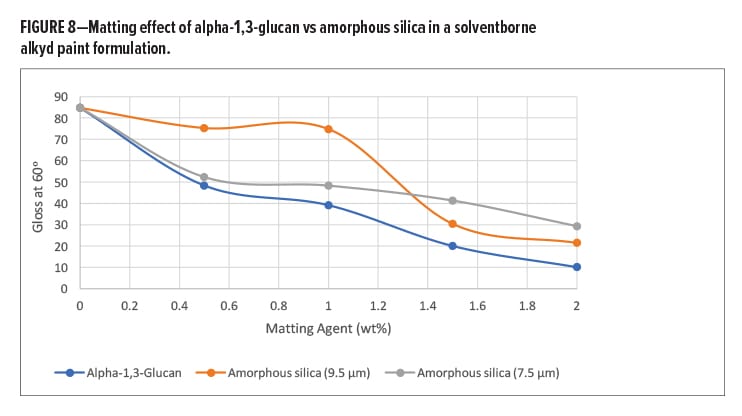
The dust-free, tack-free, and hard dry times were measured according to ISO 1517 and CSN 67 6052, respectively. The dust-free and hard dry times were unaffected upon the addition of even 2 wt% of the alpha-1,3-glucan polymer. The tack-free time was reduced by a third already at low addition levels (e.g., 0.5 wt% loading) of the alpha-1,3-glucan polymer (Table 3).
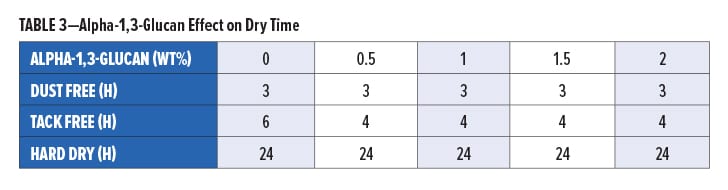
The surface hardness of the films was measured using ISO 1522, and the results are plotted in Figure 6. There is a positive correlation between the concentration of alpha-1,3-glucan in the formulation and the level of hardness of the paint film. The addition of 2 wt% alpha-1,3-glucan yields a paint with about 30% more hardness after 28 days.
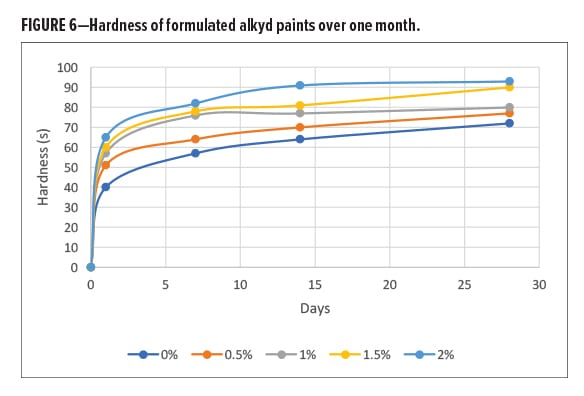
Gloss of the paint films was evaluated using ISO 2813:2014. Figure 7 shows the reduction in gloss at 60º upon the addition of alpha-1,3-glucan. At only 0.5 wt% loading the gloss was reduced by about 43%. Due to its matting effects, alpha-1,3-glucan is suitable for semi-gloss and flat paints.
The alpha-1,3-glucan was also compared against other matting agents, like amorphous silica, in the same solventborne alkyd paint formulation (Figure 8). The two grades of silica used for the formulation had a typical average particle size of 7.5 and 9.5 micron. The alpha-1,3-glucan shows a higher degree of matting compared with both grades of silica at the same loading levels.
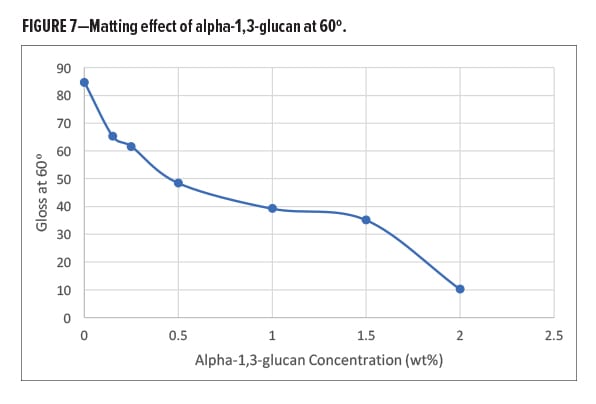
In the solventborne alkyd formulation, the alpha-1,3-glucan polysaccharide additive is effective as a multi-functional additive, allowing for the replacement option of multiple traditional additives in the paint formulation. At low addition levels, the polysaccharide reduces gloss significantly while also increasing the paint hardness, reducing the tack-free time, and increasing the paint viscosity.
Conclusions
Enzymatic polymerization is advancing as a new industrial process option to provide engineered polysaccharide materials with unique structural polymer properties and material morphology. The further development and commercialization of this bioprocess will allow access to a family of unique biomaterials that can be tailored to provide differentiated properties in numerous industrially important applications. This process is easily scalable, utilizing readily accessible, fungible, and yearly renewable feedstocks. The process is highly capital efficient and offers an attractive overall atom economy to provide materials at scale and enabling cost.
The example described here reports on work using the alpha-1,3-glucan polymer in combination with coating formulations for commercially relevant coating systems. The unique microstructure of the polysaccharide allows it to be easily formulated into various paint resins with excellent compatibility. Alpha-1,3-glucan works as a multi-functional additive in these formulations, and when re-dispersed under high shear the polysaccharide is able to modify the viscosity and impart a thixotropic rheology to the paint system. At low effective concentrations, the alpha-1,3-glucan is also able to increase the hardness of the paint and to reduce the overall dry time. Of special interest will be the ability to function as an additive that imparts gloss reduction for matting applications in these paint systems. Additional work using this material in industry-typical coating systems is currently underway and will be reported in due time.
References
- https://www.ellenmacarthurfoundation.org/publications/the-new-plastics-economy-rethinking-the-future-of-plastics-catalysing-action.
- van Haveren, J., Oostveen, E.A., Miccichè, F., Noordover, B.A.J., Koning, C.E., van Benthem, R.A.T.M., Frissen, A.E., and Weijnen, J.G.J., “Alkyd Review: Resins and Additives for Powder Coatings and Alkyd Paints, Based on Renewable Resources,” J. Coat. Technol. Res., 4 (2) 177–186 (2007).
- Christian, H.-D., “Novel Matting Agent for Low Gloss,” PPCJ, Polym. Paint Colour J. (1357-731X), 200 (4554), p. 14 (2010).
- Nasadova, J. “Driers suitable for high solids alkyd paints II,” Sbornik Prispevku – Mezinarodni Konference o Naterovych Hmotach, 44th, Pardubice, Czech Republic, p. 83, May 20-22, 2013.
- Lenz, P., Hans, M., and Luttikhedde, H., Farbe + Lack, Vol. 119, Issue 4,
pp. 140-143 (2013). - Shiro Kobayashi, et al., “In Vitro Synthesis of Cellulose and Related Polysaccharides,” Prog. Polym. Sci., 26, 1525-1560 (2001).
- U.S. Patent 20150232819; Paullin, J.L., Payne, M.S., Dennes, T.J., Brun, Y., Nambiar, R., and Scholz, T. (2015).
- Sakarin Puanglek, et al., “In Vitro Synthesis of Linear α-1,3-glucan and Chemical Modification to Ester Derivatives Exhibiting Outstanding Thermal Properties,” Sci. Reports, 6:30479 (2016).
- Remaud-Simeon, M., Albenne, C., Joucia, G., Fabre, E., Bozonnet, S., Pizzut, S., Escalier, P., Potocki-Veronese, G., Monsan, P., ACS Symposium Series, Vol. 849, Issue Oligosaccharides in Food and Agriculture, pp 90-103 (2003).
- The high slurry viscosity suggests particles with high hydrodynamic radius, much higher compared with the hydrodynamic radius of a typical solid, spherical particle. This feature is also in line with the efficient matting effect we obtain in the example coatings. Direct surface area measurement is complicated by the capillary collapse induced by drying [see, for example, Beaumont, M., Kondor, A., Plappert, S., et al., Cellulose 24: 435 (2017)]. The increase of viscosity observed with the enzymatically polymerized polysaccharide in this example is comparable to other high surface area particle systems, e.g., fumed silica (for example, Aerosil™ 200); and by inference, both types of particles show comparable surface area.
- The alpha-1,3-glucan has been described structurally as isolated from fungi cell walls in which it takes on a structural function. See, for example: J. Chem. Soc., 2592-2594 (1952); also, the similarity of this polysaccharide with cellulose was recognized and discussed, e.g., ACS Symposium Series, Vol. 260, Chapter 3, pp 43–59, 1984; further work by DuPont described the identification and use of this polysaccharide, e.g., Nichols, S. et al., Transgenic Res., Vol. 16, (4) pp. 467-78; O’Brien, J. U.S. Patent 7000000.
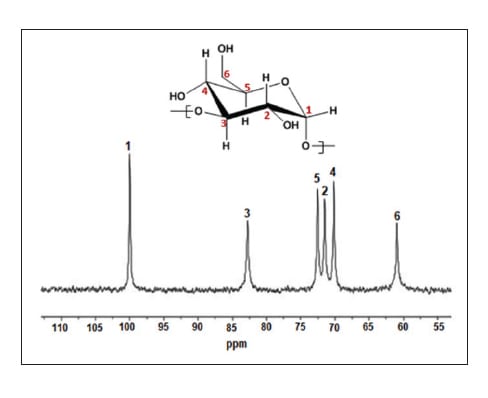
- The stereo regular nature of the isolated alpha-1,3-glucan was confirmed using a series of analytical methods ranging from chromatography and NMR analysis. For example, some details are provided here: 13C NMR experiments were performed on a Bruker Avance I NMR spectrometer operating at 500.13 MHz for 1H and 125.75 MHz for 13C. The NMR is equipped with a 5-mm carbon optimized helium cooled cryoprobe. 1D carbon NMR data was collected with inverse-gated 1H decoupling, 4096 transients, 1.5 sec acquisition time, and d1 of 5 sec. The FID was processed with Bruker Topspin software. NMR samples were prepared from ~7–10 mg of polysaccharide solid material, stirred while adding a solution of 3 wt% LiCl in DMSO-d6. Sample was stirred at 80C overnight. Alpha-1,3-Glucan 13C NMR (3 wt% LiCl/DMSO-d6, δppm): 100.17 (CH, C1); 82.49 (CH, C3); 72.62 (CH, C5); 71.58 (CH, C2); 70.1 (CH, C4); 60.74 (CH2, C6)

- U.S. Patent 7000000, Obrien, J., Jan. 25, 1999.
This paper received the American Coatings Best Paper AWARD at the 2018 American Coatings CONFERENCE, April 9–11, in Indianapolis, IN.
CoatingsTech | Vol. 14, No. 5 | May 2018