The utility of a high-throughput, spectrophotometric assay in screening over 23 enzyme and peptide-based additives against bacterial coating spoilage agents was evaluated. Candidate additives were then evaluated using ASTM D2574 for in-can coating spoilage challenges, and additives that may be used to substitute for traditional biocides were identified that eliminated recoverable bacterial growth.
Introduction
Traditional biocides approved for in-can and in-film preservation, as well as antifouling activity, (e.g., alkylating agents and crosslinkers) can have direct impact on humans, while others such as the isothiazolinones may cause sensitization following continued exposure.1-3 Such health risks and potential environmental impacts have prompted many countries to restrict the levels of use and/or require special labeling of various chemical preservatives.1-5 Combined with the trend towards the use of lower levels of volatile organic compounds (VOCs) that have preservative qualities, there is a rapidly increasing demand for development of novel preservatives that are highly effective, economically reasonable, and present minimal regulatory hurdles.6-8
Enzymes, peptides, and natural product small molecules have the potential to overcome some of the challenges associated with traditional biocides, and have been the subject of increasing interest in food and materials preservation.9-18 An extensive technology portfolio involving antimicrobial peptides (AMPs) and enzymes in coatings and materials, chiefly those exhibiting in-film efficacy, has been developed.12-17 AMPs may be able to provide a novel approach to in-can coating preservation based on principles used by organisms to protect themselves against microbial invasion. Various bio-based molecules that target different components of the microbial cell, including enzymes that degrade the cell wall (lysozyme), glycocalyx or biofilm (alginate lyase), or that generate reactive oxygen (glucose oxidase) can then be used in combination with AMPs to disrupt the cellular function synergistically. Recently, Reactive Surfaces was asked to use its bio-based biocides to completely replace traditional in-can preservatives in a well-known commercial internal architectural coating, the proprietary study, which is extended and discussed here.
The time and crude nature of techniques that have been used historically can make developing and screening of novel agents for coating preservation difficult and not data-intensive enough to detect useful trends in biocidal effects. One goal of the current study is to use modern microbiological screening methods for selection of candidates. We chose a rapid cell viability assay that quantitatively measures the metabolic ability of living cells to reduce the tetrazolium dye XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) to a colored product that is spectrophotometrically measured at 492 nm.19 This technique allows screening of candidate biocides in-can and in-film using very large arrays (of concentrations, combinations, microbial strains, coatings formulations, etc.). This preliminary screening allows quick down selection of biomolecules that inhibit metabolic activity for subsequent correlation with the traditional ASTM D2574 “Standard Test Method for Resistance of Emulsion Coatings in the Container to Attack by Microorganisms” microbiological assay when tested alone or combined with traditional chemical biocides. Such a “bookend” approach can then be used to evaluate the ultimate efficacy of antimicrobial formulations, providing the formulation chemist and microbiologist with the largest and most complete database from which to control microbes from raw materials introduction, through production, into the marketed container, and ultimately into dry films.
Materials and Methods
Reagents and Bacterial Strains
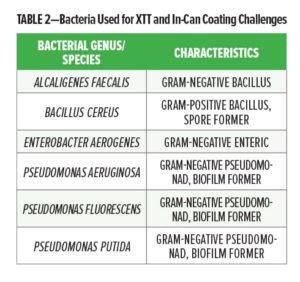
Glucose oxidase, alginate lyase, nisin (2.5%), α-amylase, β-glucosidase, β-mannosidase, β-glucanase, amyloglucosidase, cellulose, trypsin, pectinase, cinnamaldehyde, citral, and protease were obtained from Sigma-Aldrich (St. Louis, MO). Lysozyme was obtained from Bio-Cat (Troy, VA). Peroxidase was obtained from TCI America (Portland, OR). Chymotrypsin was obtained from MP Biomedicals (Santa Anna, CA). OPDtox™ and the peptides AMP-6, AMP-7, and AMP-LKLK were obtained from Reactive Surfaces, Ltd. (Austin, TX). Monolaurin was obtained by grinding Lauricidin® pellets from Med-Chem Labs, Inc. (Goodyear, AZ) into a fine powder. XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide)) was obtained from Biotium (Fremont, CA). Bacterial broth cultures used either SelenoMetTM (SM) minimal media from Molecular Dimensions (Altamonte Springs, FL) or Bacto Tryptic Soy Broth (TSB) from Becton, Dickinson, and Co. (Sparks, MD). All growth media used Difco Tryptic Soy Agar (TSA) from Becton, Dickinson, and Co. (Sparks, MD). Pseudomonas aeruginosa (#155250A), Pseudomonas putida (#155265), Pseudomonas fluorescens (#155255), Alcaligenes faecalis (#154835A), Bacillus cereus (#154870) and Enterobacter aerogenes (#155030) cultures were obtained from Carolina Biological Supply (Burlington, NC).
Measuring Cell Viability Using XTT
Pseudomonas aeruginosa, Pseudomonas putida, Pseudomonas fluorescens, Alcaligenes faecalis, Bacillus cereus, and Enterobacter aerogenes cultures were grown in 5 mL SM or TSB broth overnight at 30°C with agitation, then diluted 1:10 in SM or TSB for assays measuring the effect of single additives of the growth of individual strains. In experiments looking at the effects of additives on mixed cultures, 1 mL from each overnight culture was first combined in a sterile tube, and the microbial mixture was diluted 1:10 with SM media. A stock of menadione was prepared at a concentration of 1.7 mg/mL in acetone, and was diluted 1:120 into a solution of 1 mg/mL XTT in PBS (filter-sterilized with 0.45 mm nylon filter (Fisher Scientific)) immediately before setting up the assay. Stock solutions were prepared in dimethyl sulfoxide (DMSO) for monolaurin, cinnamaldehyde, and citral, or sterile water for all other additives so that the stock concentration was 20X the final test concentration. Each well in a 96-well microplate, in triplicate, received 10 mL of the additive to be tested, 20 mL of the XTT/menadione solution, and 100 mL of diluted cells, with the remainder consisting of growth media so that the final volume for each well was 200 mL. Wells containing diluted cells without additives (or with 10 mL DMSO for additives requiring DMSO for solubility) were included as negative controls. The absorbance at 492 nm was measured before and after incubation at 30°C for 20 h. The percent increase in absorbance and percent reduction in metabolism was calculated for each treatment as described.23 Testing was performed in triplicate using three different concentrations of the bio-based additives.
Coating Challenges
Coating challenges were conducted as described in the ASTM International Standard procedure D2574-16.24 Pseudomonas aeruginosa, Pseudomonas putida, Pseudomonas fluorescens, Alcaligenes faecalis, Bacillus cereus, and Enterobacter aerogenes cultures were grown in 5 mL TSB broth for 24 h at 30°C with agitation. Sterile inoculation loops were used to pass a loop-full of each culture into new 5 mL TSB broths, which were incubated for 24 h at 30°C with agitation. The cultures were passed and incubated again for 24 h. Coating samples were prepared by hand mixing additives into 25 mL of acrylic latex coating using sterile glass rods. Sterile swabs were used to sample each coating and streak TSA plates to ensure that the coatings were sterile prior to inoculation. One mL from each broth culture was combined into a sterile tube and mixed well immediately prior to inoculation of the coating. The coating samples were inoculated with either 25 mL or 250 mL of the microbial mixture, and the coatings were incubated at 30°C for the duration of the test. Sterile swabs were used to sample each coating and streak duplicate TSA plates on days 1, 3, 5, and 7 following inoculation. The TSA plates were incubated at 30°C for one week, after which the amount of bacterial recovery from the coating was scored as described in the ASTM standard procedure:
0—no bacterial recovery
1—trace contamination (1 to 9 colonies)
2—light contamination (10 to 99 colonies)
3—moderate contamination (> 100 distinct colonies)
4—heavy contamination (continuous smear of growth, colonies have grown together and are indistinguishable)
Results
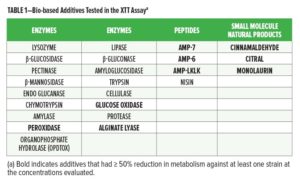
From an initial panel of 30 enzymes, peptides, and small molecule natural products, 23 listed in Table 1 were selected for evaluation. These were screened against individual members of a microbial contamination panel listed in Table 2 using the XTT assay. Nine of the 23 bio-based additives were found to reduce cellular metabolism in the XTT assay by ≥ 50% (bold in Table 1).
 Following the screening of individual strains, the XTT assay was used to evaluate effectiveness of individual additives or additive combinations against a mixed inoculum of all six test strains. This was similar to the inoculation procedure used in ASTM D2574 where all strains are grown separately and then inoculated as a mixture into the coating. Several complex mixtures, particularly with those bio-based additives that target different molecular components of the cell, were analyzed for additive or synergistic effects. Figure 1 shows the results of the mixed bacterial inoculum XTT testing.
Following the screening of individual strains, the XTT assay was used to evaluate effectiveness of individual additives or additive combinations against a mixed inoculum of all six test strains. This was similar to the inoculation procedure used in ASTM D2574 where all strains are grown separately and then inoculated as a mixture into the coating. Several complex mixtures, particularly with those bio-based additives that target different molecular components of the cell, were analyzed for additive or synergistic effects. Figure 1 shows the results of the mixed bacterial inoculum XTT testing.
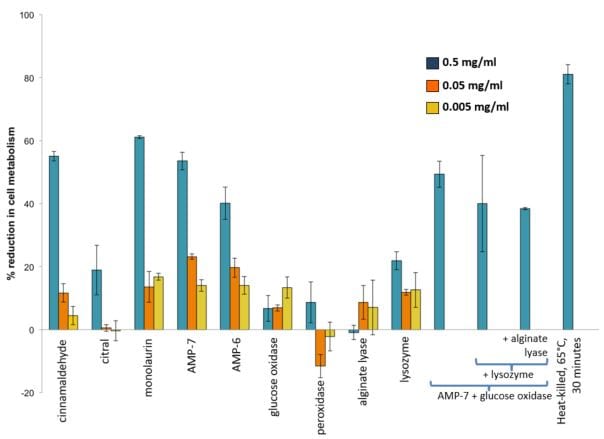
FIGURE 1—Effects of various bio-based additives, alone and in combination, on the growth of a mixture in equal parts of A. faecalis, B. cereus, E. aerogenes, P. aeruginosa, P. fluorescens, and P. putida. The blue bars represent the highest concentration tested 0.5 mg/mL, the orange bars represent the middle concentration tested of 0.05 mg/mL, and the yellow bars represent the lowest concentration tested, 0.005 mg/mL, for each compound.
Several additives displayed similar trends in activity against the mixed inoculum as was seen with individual strains—with cinnamaldehyde, monolaurin, and AMP-7 showing the greatest reduction in cellular metabolism of 55%, 61%, and 54%, respectively. In addition, a combination containing AMP-7/glucose oxidase had a reduction in cellular metabolism of 49%. AMP-6, AMP-LKLK, and a complex combination of lysozyme/AMP-7/glucose oxidase/alginate lyase showed approximately 40% reduction in cellular metabolism. All other bio-based additives tested were below 25% reduction in metabolism.
Based on the XTT results, select bio-based additives and combinations were tested against the microbial test panel using direct coating challenges as described in ASTM D2574 followed by sampling for recoverable growth over seven days. A biocide-free acrylic latex negative control coating and biocide positive control acrylic latex coating containing Kathon™ LX 1.5% (final concentration 0.15 wt%) were prepared and used in this study (Table 3).
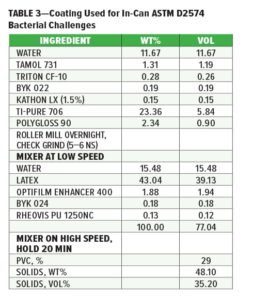
ASTM D2574 paint challenges were performed using increasing bio-based additive concentration levels from those showing greater than 50% reduction in metabolism in the XTT assay. The coating containing Kathon LX 1.5% showed efficient control of the mixed microbial test panel with a score of 0 on day 1. Cinnamaldehyde at 1 mg/mL showed gradual decline in recoverable growth, achieving a 0 rating by day 7. This rate of decline was accelerated in a combination containing 0.05 mg/mL glucose oxidase to a 0 rating by day 5. A more rapid rate of decline was also seen at higher test concentrations of cinnamaldehyde comparable to those resulting in over 80% reduction in metabolism in the mixed panel XTT assay (determined to be 89% at 5 mg/mL). In a separate experiment at the higher test concentrations, a combination containing cinnamaldehyde and glucose oxidase showed a slightly greater impact on day 1 with a 2.5 (one plate scoring a 2 and the other scoring a 3) on day 1 vs a 3 for the cinnamaldehyde coating alone, and both samples reached a 0 by day 3. At the tested concentrations, neither AMP-7, AMP-6, monolaurin, glucose oxidase alone or in combination with other bio-based additives targeting different molecular targets, decreased the score from 4 by day 7. However, in the analogous proprietary studies using different coatings formulations with or without traditional biocides and a different panel of microbial strains, these same bio-based additives demonstrated efficacy. These differences suggest to the authors that bio-based additives, much like traditional biocides, should be selected to protect a given type of coating formulation or category of raw material against the microbial challenges encountered in a particular application.
Following these encouraging results, the XTT assay was utilized to evaluate the potential for synergistic effects between bio-based additives and traditional biocides. MIT (2-methyl-4-isothiazolin-3-one) and DMDM (1,3-dihydroxymethyl-5,5-dimethylhydantoin) were tested as described above for the bio-based additives. The concentration of MIT and DMDM was kept constant at 15 ppm, either alone or in combinations with varying concentrations of bio-based additives (Figure 2).
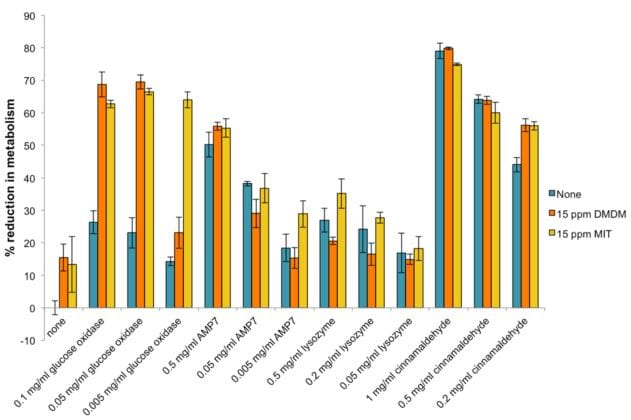
FIGURE 2—Effects of various bio-based additives, alone and in combination with traditional chemical-based biocides, on the growth of a mixture in equal parts of A. faecalis, B. cereus, E. aerogenes, P. aeruginosa, P. fluorescens, and P. putida. The blue bars represent no chemical biocide present, the orange bars represent 15 ppm DMDM present, and the yellow bars represent 15 ppm MIT present, with each bio-based additive tested.
The most dramatic impact on activity of the traditional biocides was seen with glucose oxidase. DMDM went from approximately 15% reduction in metabolism at 15 ppm to nearly 70% reduction in metabolism when combined with the two highest test concentrations of glucose oxidase (which had under 30% reduction alone). MIT increased from less than 15% reduction in metabolism alone, to greater than 60% reduction in metabolism when combined with glucose oxidase at any of the test concentrations.
Discussion
The XTT assay of single bacterial challenge microbes successfully identified bio-based enzymes, peptides, and small-molecule natural product additives that could reduce cellular metabolism by 50%, with those having highest activity defined as reducing metabolism to over 80% being comparable to heat-killed experiments where no viable cells are detectable. For the single additives, high activity against all the individual strains correlated to high activity in the mixed inoculum test results. In some cases, all members of a mixed inoculum were eliminated similarly to a traditional biocide in the same coating. In other cases, all but a single up to only a few microbial contaminants were eliminated from a mixed inoculum by use of a bio-based biocidal formulation—which results were distinguished by a lower kill-rate exhibited by the metabolic assay, and a single species lawn or isolated colonies in the standard ASTM plating assay. Species-specific differences were detected in the current study, for instance, P. putida being susceptible to more classes and concentrations of bio-based additives tested as compared to P. aeruginosa. In certain instances (not reported here), in-film antimicrobial efficacy was observed against bacterial, mold, and algal challenges of fixed films of the in-can preserved coatings. These results indicate the potential to substitute bio-based biocides for traditional biocides.
Several in-can challenge methodologies routinely used by the industry can vary in their degree of specificity, and it can be difficult to draw conclusions between them.25 For instance, a score of 2 or 3 in the ASTM could be a single colony type or multiple colony types, and does not discern if each strain is remaining viable in the coating. Even for a score of 4, visual differences in colony types present could be observed in some cases (Figure 3).
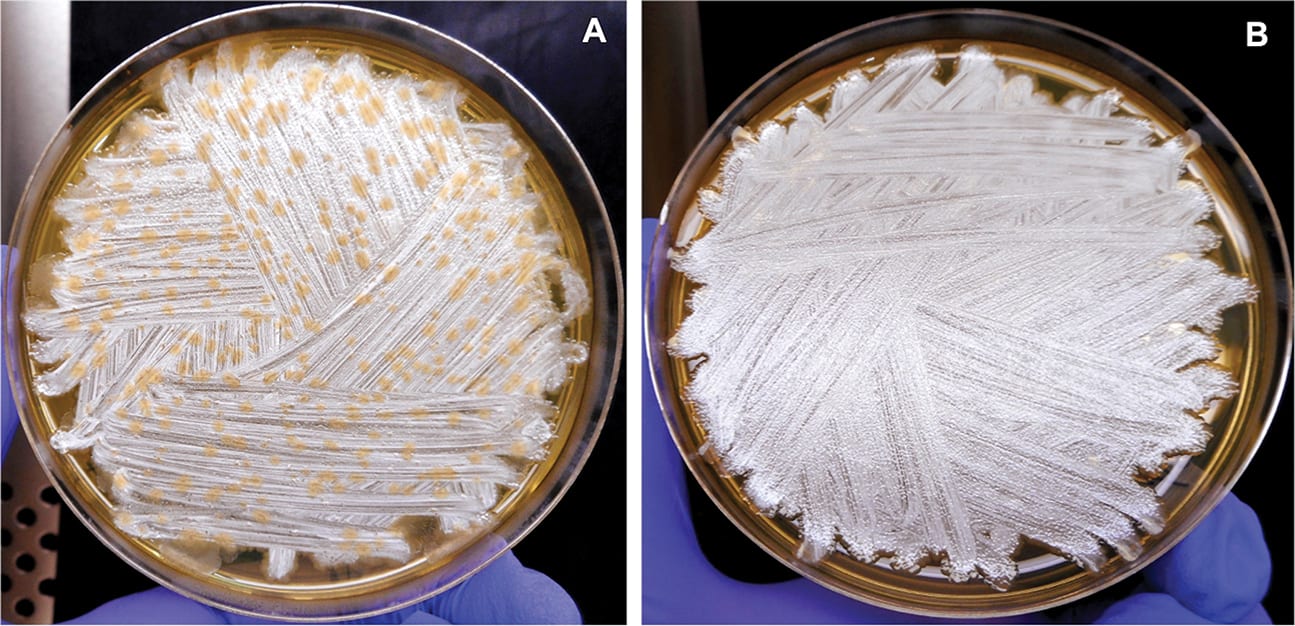
FIGURE 3—Comparison of colony types present on plates from paint samples that scored 4 on day 7. Comparison of day 7 plates from the control acrylic latex sample (A) and acrylic latex + 0.05 mg/mL glucose oxidase + 2 mg/mL dextrose (B) shows that the paint with bio-based additive, though scoring 4 according to the ASTM guidelines, had at least one fewer colony type compared to the control.
Having more data-intensive results is critical where the contamination, as it almost always is, is the result of a microbial community, not a single microbe. In a subsequent Part II, we will demonstrate and discuss this finding. In this study, comparing activities against single bacterial isolates to a mixed inoculum containing all six test bacterial strains can be used to quickly focus the preservative formulation to those contaminants that exhibit initial recalcitrance. For example, glucose oxidase and alginate lyase showed activity against one or more test strains alone, but had < 20% reduction in metabolism against the mixed inoculum. This indicates that less than complete kill was achieved for at least one of the mixed strains, allowing for continued survival and growth in the culture media. One rationale to overcome a resistant contaminant is to select disruptive properties impacting distinct cellular components to achieve synergistic effects, with a lysozyme, AMP-7, glucose oxidase, and alginate lyase combination as an example. Alginate lyase targets the extracellular polysaccharide layer, AMP-7 disrupts cellular membranes, lysozyme cleaves bacterial cell wall peptidoglycans, and glucose oxidase produces hydrogen peroxide that can induce cellular damage.26
The current lead candidates based on the XTT and ASTM data include AMPs, glucose oxidase, and lysozyme. AMPs play an important role in host defenses against microorganisms, and have demonstrated antimicrobial activity in solution and in dry-film formulations against various microorganisms, including bacteria (including Pseudomonas), fungi, algae, and viruses.12-17 Reactive Surfaces is currently undertaking EPA registration of AMP-7. Glucose oxidase and lysozyme are both listed as Generally Regarded as Safe for various intended uses and have been used in the food industry.21-22 They are produced on commercial scale and available in bulk quantities. Lysozyme is also notable for being particularly effective against Gram-positive bacteria.13
These results indicate that an initial metabolic or other rapid-throughput assay can be used to predict likelihood of success of bio-based biocides as in-can preservatives. For example, such testing shown here resulted in an 80% reduction in metabolism using the mixed inoculum translated into a complete kill in the ASTM challenge method. In addition, molecular methods are being developed to conduct direct analysis of microbial community structure in coating samples, such as rapid identification of each microbial species using DNA amplification and sequencing techniques. This will allow for real-time monitoring of individual bacterial growth patterns in the coating sample and impacts on each strain of the consortium following bio-based additive treatment.
References
1. Chan, P.K., Baldwin, R.C., Parsons, R.D., Moss, J.N., Stiratelli, R., Smith, J.M., and Hayes, A.W., “Kathon Biocide: Manifestation of Delayed Contact Dermatitis in Guinea Pigs Is Dependent on the Concentration for Induction and Challenge,” J. Invest. Dermatol., 81, No. 5, pp. 409–411 (1983).
2. Schwensen, J.F., Menné, T., Andersen, K.E., Sommerlund, M., and Johansen, J.D., “Occupations at Risk of Developing Contact Allergy to Isothiazolinones in Danish Contact Dermatitis Patients: Results from a Danish Multicentre Study,” (2009-2012), Contact Dermatitis, Nov. 771, No. 5, pp. 295-302 (2014).
3. Schwensen, J.F., Lundov, M.D., Bossi, R., Banerjee P., Giménez-Arnau, E., Lepoittevin, J.P., Lidén, C., Uter, W., Yazar, K., White, I.R., and Johansen, J.D., “Methylisothiazolinone and Benzisothiazolinone Are Widely Used in Coating: A Multicentre Study of Coatings from Five European Countries,” Contact Dermatitis, 72, No. 3, pp. 127–138 (2015).
4. Bollmann, U.E., Fernández-Calviño, D., Brandt, K.K., Storgaard, M.S., Sanderson, H., Bester, K., “Biocide Runoff from Building Facades: Degradation Kinetics in Soil,” Environ. Sci. Technol., 51, No. 7, pp. 3694–3702 (2017), Apr. 4, 51 (7), pp. 3694–3702.
5. Lundov, M.D., Kolarik, B. Bossi, R., Gunnarsen, L., and Johansen, J.D., “Emission of Isothiazolinones from Water-based Coatings,” Environ. Sci. Technol., 48, No. 12, pp. 6989–6994 (2014).
6. Cresswell, T., Richards, J.P., Glegg, G.A., and Readman, J.W., “The Impact of Legislation on the Usage and Environmental Concentrations of Irgarol 1051 in UK Coastal Waters,” Mar. Pollut. Bull., 52, No. 10, pp. 1169–1175 (2006).
7. Brinch, A., Hansen, S.F., Hartmann, N.B., and Baun, A., “EU Regulation of Nanobiocides: Challenges in Implementing the Biocidal Product Regulation (BPR),” Nanomaterials (Basel), 6, No. 2, pp. E33 (2016).
8. “White Paper: Antimicrobial Building Products Should Be Avoided Whenever Possible,”
9. Bhatia, S. and Bharti, A., “Evaluating the Antimicrobial Activity of Nisin, Lysozyme and Ethylenediaminetetraacetate Incorporated in Starch-based Active Food Packaging Film,” J. Food Sci. Technol., 52, No. 6, pp. 3504–3512 (2015).
10. Yuan, S., Wan, D., Liang, B., Pehkonen, S.O., Ting, Y.P., Neoh, K.G., Kang, E.T., “Lysozyme-coupled Poly(poly(ethylene glycol) methacrylate) – Stainless Steel Hybrid and their Antifouling and Antibacterial Surfaces,” Langmuir, 27, No. 6, pp. 2761–2774, (2011).
11. Zodrow, K.R., Schiffman, J.D., and Elimelech, M., “Biodegradable Polymer (PLGA) Coatings Featuring Cinnamaldehyde and Carvacrol Mitigate Biofilm Formation,” Langmuir, 28, No. 39, pp. 13993–13999 (2012).
12. Williams, E., Carvajal, J.C., Schwausch, M., McDaniel, C.S., Wales, M., Rawlins, J., “Nature Versus Natural: Engineered Oligopeptides that Prevent Microbial Degradation of Coatings.” 37th International Waterborne, High-Solids and Powder Coatings Symp., New Orleans, LA, 2011.
13. McDaniel, C.S., Anti-fouling Coatings and Coatings, U.S. patent application 14/097,128, July 9, 2015.
14. McDaniel, C.S., Antifungal Coatings and Coatings, U.S. patent 8,497,248, July 30, 2013.
15. McDaniel, C.S., Coating Compositions having Peptidic Antimicrobial Additives and Additives of Other Configurations, U.S. patent 8,618,066, December 31, 2013.
16. McDaniel, C.S., Antifungal Coatings and Coatings, U.S. patent 7,932,230, April 26, 2011.
17. McDaniel, C.S., Anti-fouling Coatings and Coatings, U.S. patent 7,939,500, May 10, 2011.
18. Cloutier, M., Mantovani, D., and Rosei, F., “Antibacterial Coatings: Challenges, Perspectives, and Opportunities,” Trends in Biotechnology, 33, No. 11, pp. 637–652 (2015).
19. XTT Cell Proliferation Assay Kit Instruction Manual, American Type Culture Collection, Manassas, VA, 2011.
20. Tiina, M. and Sandholm, M., “Antibacterial Effect of the Glucose Oxidase-Glucose System on Food-Poisoning Organisms,” Internat. J. Food Microbiol., 8, No.2, 165–174 (1989).
21. Lysozyme Safety Toxicology and Safety Statement, https://www.fordras.com/lysozyme-safety/ (accessed December 2017).
22. Bankar, S.B., Bule, M.V., Singhal, R.S., and Ananthanarayan, L., “Glucose Oxidase—an Overview,” Biotechnology Advances, 27, No. 4, pp. 489–501 (2009).
23. Khurram, M., Lawton, L.A., Edwards, C., Iriti, M., Hameed, A., Khan, M.A., Khan, F.A., and Rahman, S.U., “Rapid Bioassay-Guided Isolation of Antibacterial Clerodane Type Diterpenoid from Dodonaea viscosa (L.) Jaeq.” Int J Mol Sci., 16, No. 9, pp. 20290-20307 (2015).
24. “ASTM Standard D2574-16, Standard Test Method for Resistance of Emulsion Coatings in the Container to Attack by Microorganisms,” ASTM International, West Conshohocken, PA, (2016), DOI: 10.1520/D2574-16.
25. Winkowski, K., “Efficacy of In Can Preservatives,” European Coatings Journal, 1-2, pp. 87-91 (2001).
26. Hall-Stoodley, L., Costerton, J.W., Stoodley, P., “Bacterial Biofilms: From the Natural Environment to Infectious Diseases,” Nat. Rev. Microbiol., 2, No. 2, pp. 95-108 (2004).
This article was presented at the Waterborne Symposium, February 4–9, 2018, in New Orleans, LA.
*Certain of the coauthors from Reactive Surfaces and the University of Southern Mississippi received the inaugural American Coatings Award in 2008 for work leading to the research reported here. An expansion of this study will be reported in Part II discussing the in-film efficacy of proteins and peptides in packaging for enhanced food preservation.
†Tyler W. Hodges and Kyle L. Wilhelm are also affiliated with William Carey University, Hattiesburg, MS.