By A. Fream, C. Baude, and M. Secher OMNOVA Solutions, Specialty Coatings, Adhesives and Surface Treatments
In recent years, thin film intumescent coatings have become the preferred choice for the protection of structural steel. This is a result of their ability to provide a cost-effective passive fire protection solution while maintaining the aesthetic qualities of the steel, which is being demanded by more and more architects and engineers.
Over the years, multiple generations of solventborne resins have been developed that, when formulated into end-use coatings, have proven durable and effective in reactive fire protection. We have offered such solutions based on a unique manufacturing process, polymer composition, and morphology. Now, a new water-based resin technology, based on the same chemistry and expertise, has been developed to meet the fire-resistance requirements of best-in-class products.
This article describes the development of this technology, which allows formulators to achieve superior water resistance compared to current existing water-based products, when tested according to durability requirements of standards such as ETAG 018/EN 16623.
Introduction
The fire that swept through the 24-story Grenfell Tower in London in June 2017 tragically claimed 71 lives.1 Such high losses of life are very rare, despite that fact that fires in high-rise buildings are surprisingly more common than the newspaper headlines would lead one to think.
Statistics published by the National Fire Protection Association (NFPA) in the United States in 2016,2 covering the period from 2009–2013, indicate that, in the United States alone, there are more than 14,500 structure fires per year in high-rise buildings. Very few of these fires make the headlines, simply because they are not sufficiently newsworthy. Injuries and deaths from high-rise fires are very rare with an annual average of “only” 40 civilians from 14,500 fires. This highlights the exceptional nature of the Grenfell Tower fire.
The NFPA report also concludes that a major reason why risks of injury and death in high-rise buildings are low is because of the much greater use of fire protection systems in high-rise compared to low-rise buildings. Every year, devastating building fires account for approximately one percent of world GDP that literally goes up in smoke, and fires like Grenfell Tower are a constant reminder to us all that fire safety in new construction or renovation is of paramount importance. Therefore, fire protection is an important consideration of many national building codes. The amount of protection required is usually related to the height or number of stories of the building and the density of occupancy.3
Grenfell Tower was a concrete construction not requiring structural fire protection, but steel framed buildings, which are an increasingly popular means of construction today, are required to be fire protected, to protect human lives during the unfortunate event of a fire. Although steel itself does not burn, it loses strength and load-bearing capacity when exposed to temperatures above 500°C.
Intumescent coatings, unlike other forms of fire protection such as boards and sprayed cementitious systems, can be used to provide a decorative and protective finish that does not detract from the original appearance of the exposed steelwork. This aspect of enabling the architect to use the full creative design possibilities of the steel itself has led to the increasing use of intumescent fire protective coatings in the past two decades.
The efficiency of intumescent coatings for protection of structural steel has seen considerable improvement in recent years, making them more cost competitive vs other fire protection methods— another reason for their increased growth. Historically, intumescent coatings for cellulosic fire protection have been solvent-based, being universally applicable, for interior and exterior, and with film formation independent of climatic conditions, making them suitable for all climates and all seasons. However, in recent years, there has been a steady increase in the adoption of waterborne intumescent coatings in certain markets, not for legislative or regulatory reasons, but more so for comfort of application, since they are lower in odor and toxicity and, therefore, better suited for interior application.
However, with water-based intumescent coatings, little progress has been made in reducing water sensitivity, their “Achilles heel.” Consequently, waterborne products are generally limited to interior conditions.
The poor water resistance of these coatings is primarily related to the water sensitivity of the binders used to formulate them, which is, in turn, a function of their chemistry. This article addresses the development of a new polymer for use in the formulation of waterborne intumescent coatings. The new polymer can be formulated into very cost-competitive coatings and achieve up to two hours of fire resistance. Additionally, it offers significantly improved water resistance, which will extend the service use to semi-exposed and exposed environmental conditions.
Intumescent Coatings: What Are They? How Are They Formulated? How Do They Work?
Intumescent (reactive) coatings are coatings that react under the influence of fire and swell in a controlled manner to many times their original thickness, producing an insulating carbonaceous char or foam that protects the substrate from the effects of the fire.
Intumescence is generally accomplished with a minimum of three components: a source of carbon [typically pentaerythritol (PER) or dipentaerythritol, (DIPER)], a blowing agent [typically melamine (MEL) and solid chlorinated paraffin (CP)], and a source of mineral acid catalyst [typically ammonium polyphosphate (APP)].
The basic technology of intumescent coatings has changed little over the past 40 years. However, our knowledge of the chemistry has increased significantly, and the technology of the key ingredients has evolved, and continues to evolve, to keep pace with the needs of the market.
When an intumescent coating is subjected to heat, a series of chemical reactions occurs: the APP decomposes to produce phosphoric acid; the phosphoric acid causes dehydration of the PER or DIPER to produce a carbon char; the blowing agent decomposes, releasing nonflammable gases, which cause the carbon char to foam, thus producing a meringue-like structure that is a highly effective insulator against heat. Also critical to the formulation of an intumescent coating is titanium dioxide (TiO2), which not only provides the usual properties of color and opacity, but also takes place in the intumescence process.4 The very fine particles of TiO2 act as nucleating agents or bubble growth sites for the intumescent foam. Furthermore, at temperatures of around 600°C, the TiO2 reacts with APP to form titanium pyrophosphate, a refractory material, which stabilizes the insulating foam at high temperatures, when most of the carbon has oxidized and burned off.
One final key ingredient is the binder, which not only has the typical role of a paint binder but also has to melt over the right temperature range, with the right sort of rheology, and trap the gases within the melting/expanding char so as to produce uniform cellular structure providing good thermal insulation.
The Importance of the Resin Binder in Intumescence
The role of the binder has been studied in much depth in recent years.5-8 There is no doubt about the importance of the binder in an intumescent coating. Indeed, in Europe, in the CEPE “Guidance to a quality control fire test regime for intumescent coatings,”9 the probability of effect on fire protection is stated as “certain” and the fire test level required if the binder is changed in a formulation is “5” (the highest).
Standards for Fire Protection Coatings
Intumescent coatings, in common with other forms of fire protection, are required to be certified for use and as such are tested to rigorous standards to determine fire resistance time. The evaluation of the resistance to fire determines how long the elements coated with the fire protective materials will resist the fire while maintaining the design properties in terms of structural stability or load bearing capacity.
The market for cellulosic fire protection is very regional, and this is reflected by the many national standards that exist globally for testing of fire protective coatings, as shown in Table 1. For example, in the European Union, the performance of an intumescent (or reactive coating, as it is often referred) in terms of its resistance to a cellulosic fire is determined in accordance with CEN fire resistance test methods, which are currently EN 13381-6, EN 13381-8, and prEN 13381-9 (EN13381=test methods for determining the contribution to the fire resistance of structural members). The fire stability of a structural steel element protected with an intumescent or reactive coating is rated in minutes.
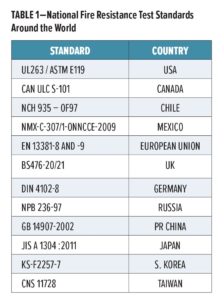
Historically, many of these test standards only covered fire testing. However, in recent years, regions such as Europe and the United States have now incorporated aspects of durability testing, together with the fire testing. It is very important that the performance of fire protective reactive coating systems, under service conditions, does not deteriorate during their assumed intended working life so as to affect significantly the performance of the products, especially the protective effects in case of fire.
The durability and reliability of the fire protection system are very important, because it is essential that the performance of these reactive fire protection systems be maintained under service conditions. The key ingredients of intumescent coatings, APP, PER, and MEL, all exhibit slight solubility in water, so it is perhaps not surprising that durability has become a topic of concern for the industry.10-12
As the fire testing standards such as EN 13381 are concerned only with fire testing, and cover no aspect of durability, new additional standards have been developed and introduced in recent years to cover this aspect, for example, UL243113 for the United States and EN16623/ETAG 01814,15 for Europe.
Durability Standards
$1
United States: The UL2431 standard is intended to provide a means to measure the ability of fire-resistive materials to retain their fire-resistive properties after being subjected to various conditioning environments. The original UL2431 standard was designed for durability testing of spray-applied fire-resistive materials (SFRM–cementitious type fireproofing) but has been revised in the past few years to also cover IFRM (intumescent fire-resistive materials).
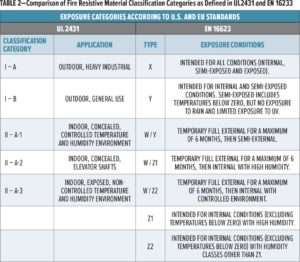
The standard defines five classification categories, depending on the exposure type and severity of exposure (see Table 2), to which the fire-resistive material will be subjected during service. The standard then defines the tests to be used for accelerated testing of the coatings for each classification category.
Europe: The EN 16623 standard, first published in 2015 and currently under review, is expected to form the basis for a future harmonized European standard (hEN) for intumescent fire protection coatings for metallic substrates. The ultimate objective is to establish a level playing field for reactive (intumescent) coatings for cellulosic fire situations, but it may take several years before a finalized version appears.
The classification system used in EN 16623 was originally devised as part of the European Technical Assessment system for intumescent (reactive) coatings and is included in ETAG 018. Categories related to exposure conditions within the standard, which is a refinement of those listed in ETAG018-2, are also outlined in Table 2, in comparison with the U.S. categories. The main evolution of the categories between those in the original ETAG 018 and those described in EN 16623 is the addition of the three “W” categories, which cover off-site (factory applied) intumescent coated structural steel components that may be temporarily exposed to the weather for up to six months after delivery to the construction site.
In parallel with the UL2431 standard, each exposure type has its own well-defined accelerated testing regime.

Waterborne Intumescent Coatings
Today, in the cellulosic fire segment of the market [“cellulosic” = fire event fueled by cellulosic materials (paper, card, wood, textiles, etc.) as opposed to “hydrocarbon” = fire event fueled by hydrocarbons
(gasoline, diesel, etc.)], both solvent-based and waterborne intumescent products exist. Although waterborne intumescents have experienced important growth in recent years, especially for on-site application, their use today is still mainly limited to interior environments, because they have a number of well-known weaknesses, not least of which is poor “durability,” related to their high sensitivity to humidity and water.
Additionally, waterborne intumescent coatings tend to have a narrower application window and can be slow drying in thick films, which limits the maximum application thickness per coat. Additional issues sometimes encountered include poor in-can storage stability (especially at low temperature, ca. 5°C), poor freeze-thaw resistance, and occasionally overcoating issues (due to re-solubilization of the intumescent coating by waterborne topcoats).
Current Binder Technology for Waterborne Intumescent Coatings
The vast majority of commercial, waterborne intumescent coatings are based on vinyl acetate (VA) copolymers, most commonly VA/vinyl versatate (VV), VA/ethylene/VV, or VA/acrylic polymers. There is a very good reason for this: VA is relatively low cost and is technically ideal for intumescence, because it is self-charring. The thermal degradation and char forming characteristics of VA are related directly to the chemistry of vinyl acetate copolymers: they contain oxygen, and have a tendency to dehydrate and form double bonds when heated (charring is a chemical process of incomplete combustion of a solid).
PVAc (polyvinylacetate homopolymer) is very water sensitive because the vinyl acetate group is easily hydrolyzed. Because of this, VA is usually copolymerized with other monomers, such as VV or acrylic, which offers steric protection of the vulnerable vinyl acetate group from hydrolysis. Although the water resistance of such copolymers can be improved compared to PVAc homopolymers, it is a fact of life that the higher the proportion of hydrolysis-resistant monomer that is incorporated into the polymer, the better is the water resistance. However, the thermal degradation characteristics are negatively affected and intumescent properties are much reduced.
The high-water sensitivity of current waterborne intumescent coatings can be clearly demonstrated by a simple immersion test in water. After less than half an hour, the coating swells, softens, and blisters, and its intumescent performance is considerably reduced because of loss of water-soluble intumescent ingredients. By comparison, a solventborne intumescent will resist more than five hours, with no blistering or loss of intumescent performance.
This weakness may not appear to be important, since many intumescent coatings are designed for service in dry, interior environments. However, it is important to note that although a reactive (intumescent) coating system may be intended for internal use only, the construction process may result in the coating system being subjected to exposed conditions for a period before the building envelope is closed. In this case, either special provisions need to be made to temporarily protect the exposed reactive coatings, or the reactive coating needs to be evaluated as if it were to be used for exposed applications, (the “W” categories of EN 16623).
New Binder for Waterborne Intumescent Coatings
$1
Seeing no way around the vinyl acetate copolymer performance/water sensitivity issues, we decided to base our new binder on the tried and trusted styrene-acrylic technology that has been the mainstay of solventborne reactive (intumescent) coatings for the past 40 years, and which is well-known for its excellent resistance to water. The advantage of this approach, therefore, is to develop a binder to enable the formulation of durable water-based intumescent coatings.
A new binder (referred to here as HPL211) has been developed specifically for the formulation of waterborne intumescent coatings. It is a styrene-acrylic emulsion copolymer, designed without the use of APEO surfactants and formaldehyde, that fully conforms to the current REACH requirements. The monomer composition has been adjusted to obtain the same good melt characteristics, and synergy with other standard intumescent ingredients (APP/PER/MEL), of the traditional solventborne resins, which results in an excellent and uniform char consistency. Char structure is one of the main parameters of effective insulation. A honeycomb char structure with uniform, dense, and regular cells, without micro-cracks or large voids, provides the best insulation properties.16
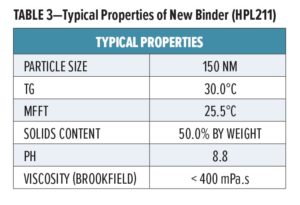
Typical physical properties of the new binder are presented in Table 3.

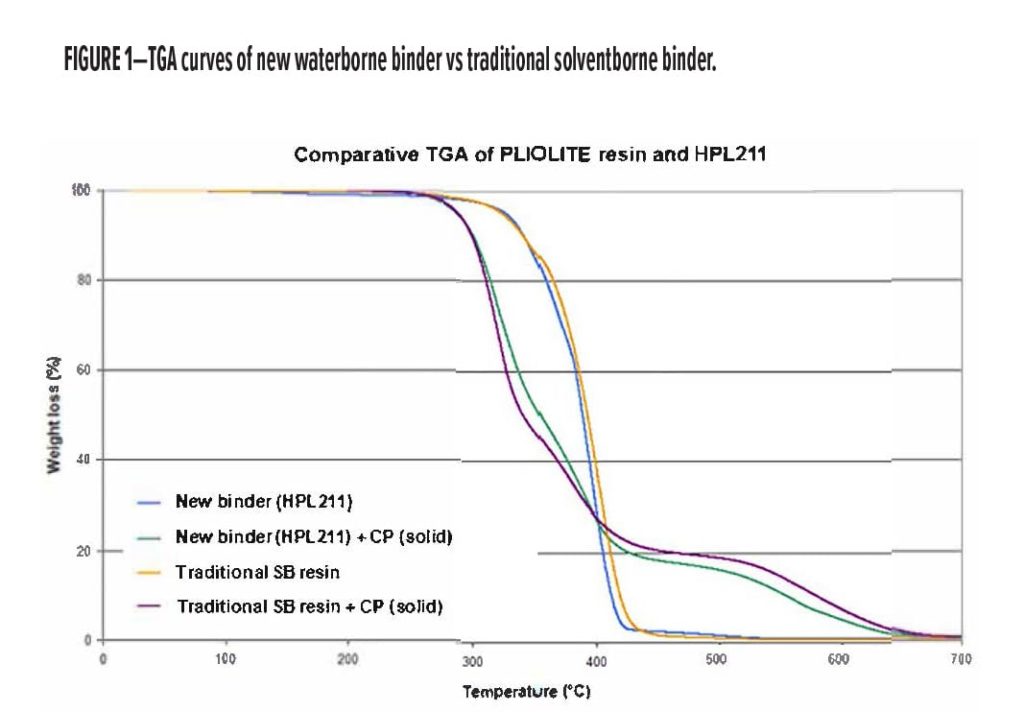
The new binder has been designed to mimic the thermal degradation of our company’s solventborne resins that have been widely used in intumescent coating formulations for many years. Degradation profiles of the new binder and traditional solventborne resin have been compared by thermal gravimetric analysis, as shown in Figure 1. It is clearly evident that the two binders begin to decompose over the same temperature range both for the resins alone and in a blend with solid chlorinated paraffin (CP), which is typically used in solventborne formulations. Moreover, decomposition occurs over the same range of temperature as the decomposition of the standard intumescent ingredients such as PER, MEL and APP, which makes for excellent intumescence and char formation of the coating.

Formulation Approach for New Waterborne Binder
$1
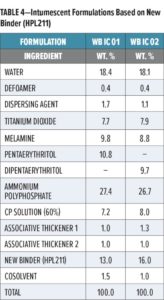
Two formulations have been developed with binder HPL211:
- A “Standard” formulation (referenced as WB IC 01, designed to provide excellent fire protection at competitive cost for EN 16623 durability categories Z1, Z2, and Y, or UL 2431 categories II A-1 to A-3. This formulation uses pentaerythritol (PER) as the carbon source (carbonific). PER has a relatively high-water solubility (5.25% according to the typical value given by the manufacturer, Charmor) but gives optimized char expansion.
- A “Premium” formulation (referenced as WB IC 02) designed to provide excellent water resistance and fire protection for all durability categories [Z1 through X (depending on topcoat used)]. This formulation uses dipentaerythritol (DIPER), which exhibits low water solubility (0.22% according to typical value from the manufacturer) and allows for the optimization of water resistance.
These two formulations, prepared by high speed dispersion, are based on the recipe structures shown in Table 4.

Experimental
Testing Protocol for Evaluation of New Binder
As fire resistance is the most critical test, laboratory evaluation begins with this step, using an indicative evaluation of fire resistance of the coating on a vertical steel plate in a small-scale laboratory furnace. This type of test is on a much smaller scale than the full-scale tests carried out by independent test laboratories for purposes of certification of fire protection products. Nevertheless, small-scale plate tests are commonly used for assessment of primer compatibility and durability tests, because even though small-scale, this kind of evaluation can give a lot of important information on the efficiency of the insulation, char consistency, and char thickness.
Fire Resistance of Intumescent Coatings in a Small-Scale Furnace
Cellulosic fire resistance tests on structural steel, such as those mentioned in Table 1, generally use a standard heating regime that follows the ISO 834 curve. The temperature development of the ISO 834 curve is described by the equation: T=20+345*LOG (8*t+1), which means that the test furnace attains a temperature of around 950°C after 60 min. An uncoated steel section placed in the furnace will gradually heat up, the lag between the furnace temperature and the temperature of the steel being related to the heat capacity, or massiveness of the steel, which is expressed as the section factor (Hp/A m-1). This is the ratio of the exposed perimeter of the steel section to the cross-sectional area of the steel: the more massive the steel section, the lower the Hp/A, the more heat it can absorb, and the longer it takes to attain the “failure” temperature (typically > 500°C). In other words, the higher inherent fire resistance the steel section has, the less fire protection it requires.
When a steel section coated with intumescent is exposed to the same furnace conditions, the steel heats up. Once the coating has intumesced to form a protective insulating layer, the rate at which the temperature of the steel increases is considerably slowed down [seen as an inflection on the temperature/time curve of the steel section (Figure 2)] until eventually it reaches the failure temperature. The time it takes to reach the failure temperature is the fire resistance time of the coating.
For the small-scale furnace tests, coatings are applied at a dry film thickness of 1 mm, usually by brush, to 5 mm thick mild steel panels of dimensions 200 x 300 mm. Two K-thermocouples are attached to bolts welded to the back of the panels, allowing the back panel temperature to be recorded. The furnace is heated by means of two propane burners, under computer control. The heating regime of the furnace is designed to follow the ISO 834 temperature/time curve for cellulosic fire. The insulation ability (fire resistance) of the intumescent coating is assessed as the average time for the two thermocouples to reach 500°C. Thickness and consistency of the resulting char are also recorded.

Durability Test
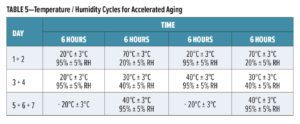
Accelerated aging tests for evaluation of durability are carried out in accordance with the procedures described in ETAG 018-2 and EN 16623-2015. For durability conditions X and W, painted panels are exposed to UV and water cycles carried out in a Q-UV cabinet following the conditions of EN ISO 16474-3:2013, Table 4, Cycle 2 with a cycle of five hours of exposure to UV-A and 50°C, followed by one hour of water spray.
For Y conditions, painted panels are subjected to accelerated aging comprising temperature and humidity changes as described in Table 5. The test is carried out in a climatic chamber that offers temperature and humidity control over 14 days, thus two times the cycle shown in Table 5.

Results and Discussion
In the first part of the study, the binder HPL211 was compared to a VA-VV latex recommended for intumescent coatings in formulation WB IC 02 at the same binder level. The water soak test and exposure to W and Y conditions were assessed comparatively for the two technologies. In the second part of the study, fire resistance of the formulation WB IC 01, based on HPL211 was compared to commercial intumescent coatings, both solventborne and waterborne systems.
Comparative Evaluation of PHL211 with Commercial VA-VV Latex
Water Soak Resistance
$1
Panels painted with intumescent paints were half-immersed in water over three hours. After drying, coated panels were subjected to heating according to the ISO 834 curve over 10 min.

The photos in Figure 3 demonstrate quite clearly the superior water resistance of HPL211 compared to the commercial VA-VV latex as the paint film of the HPL211 formulation was not visually affected by water immersion, while that of the VA-VV latex showed serious blistering and loss of adhesion. Furthermore, the coating based on HPL211 maintained its intumescent properties, while that based on the VA-VV latex no longer showed any expansion on the part that had been immersed.
W Conditions Exposure
Mild steel Q-panels painted with the two paints were exposed to W conditions in Q-UV over five days. Panels were visually assessed after the accelerated aging cycles (Figure 4).

Whereas the coating formulation WB IC 02 based on HPL211 exhibited no defect, the same formulation with VA-VV latex showed cracking and blistering due to water absorption and high-water sensitivity of this binder.
Y Conditions Exposure
$1
Coatings were applied at a dry film thickness of 1 mm on steel panels for assessment in the small-scale furnace. After one week of drying at ambient temperature and one week at 70°C, the panels were submitted to Y conditions cycling over two weeks. After three days recovery at ambient temperature, fire resistance was assessed. Results are presented in Table 6.

Both paints fulfill the requirement for Y conditions since the fire resistance after exposure is within 85% of the fire resistance of the non-exposed panel.
Although formulation with VA-VV latex and pentaerythritol shows normally good intumescence properties and fire resistance, the formulation using dipentaerythritol, which is used for durable coatings, showed a poor quality char with low expansion and large voids.
The formulation with the binder HPL211 gives good fire resistance even after Y exposure conditions, confirming, therefore, the good suitability of this product for durable intumescent coatings.
Comparative Evaluation of HPL211 Formulation with Commercial Products
The formulation WB IC 01 was compared to commercial water- and solventborne intumescent coatings obtained from the European market. Fire resistance was assessed on steel panels coated with 1 mm of intumescent coating. Results are presented in Table 7.

The formulation WB IC 01 based on HPL211 demonstrates very good expansion and fire resistance, similar to, or better than, that of best-in-class intumescent coatings, as shown in Figure 5.
Moreover, the char structure produced by the formulation

(WB IC 01) is very consistent: uniform with small cells and crispy texture, which is, as discussed previously, the ideal structure for thermal insulation. Despite very good fire resistance and expansion, the chars formed by conventional waterborne intumescent coatings exhibit a softer, more powdery aspect, which is not an ideal consistency as far as “stickability” is concerned, since the environment within a large-scale test furnace as used for fire assessments can present a lot of turbulence.
Further formulation and testing has highlighted the excellent pigment binding capability of the new binder, and the ability to give acceptable fire resistance in the small-scale furnace test at very low binder levels, while still maintaining a firm char structure and good “stickability.” This means that if water resistance is not an important criterion for the product (e.g., for interior exposure conditions), the new binder is capable of giving very economical formulations.
Conclusions
Structural steel design is a vital component of modern architecture. Steel is a versatile building material, and its high load-bearing capacity facilitates structures with wide spans that integrate perfectly into building designs and accentuate specific architectural features. Building regulations dictate that consideration be given to safety aspects of the construction, and that sometimes may appear to conflict with the modern, ornate style of architecture. Intumescent coating systems are perfectly suited to resolving this conflict: through their corrosion and fire protection, they cost-effectively fulfil all the technical requirements and, at the same time, accentuate the aesthetic appearance of a steel structure.
Traditionally, water-based intumescent coatings based on VA polymers or copolymers exhibit good intumescence properties thanks to their thermal degradation behavior, but demonstrate poor water resistance linked to their chemistry.
A new water-based binder has been developed based on the same chemistry and expertise as our company’s well-known solventborne resins for intumescent coatings. This binder can be used to formulate products that have best-in-class fire resistance performance and that meet the demands of the new durability testing standard, like EN 16623 and UL2431.
The superior water resistance of the binder HPL211, compared to current existing water-based technology, has been demonstrated using water soak and durability tests according to ETAG 018/EN 16623 in this study. The better compatibility with intumescent raw materials for durable intumescent coatings has also been evidenced in a typical water-based formulation. The high pigment binding power of the new binder gives the opportunity to formulate very competitive intumescent formulations where high levels of durability are not required.
Acknowledgments
The authors would like to thank Cécile Fonteneau, who carried out the polymerization work for this project.
References
$1
- Grenfell Tower Fire, e.g., https://en.wikipedia.org/wiki/Grenfell_Tower_fire.
- Ahrens, M., “High-Rise Building Fires,” National Fire Protection Association (NFPA), Nov. 2016.
- Morris, P., “Fire Protection of Structural Steel Using Intumescent Coatings,” Fire Safety Professional, Summer 2005.
- Duquesne, S. and Bourbigot, S., in Fire Retardancy of Polymeric Materials, 2nd Ed., Wilkie, C. and Morgan, A. (Eds.), CRC Press, Boca Raton, FL, 254-255, 2009.
- Duquesne, S., Magnet, S., Jama, C. and Delobel, R., ”Intumescent Paints,” Fire Protective Coatings for Metallic Substrates, European Materials Research Society Spring Meeting, Strasbourg, June 10-13, 2003.
- Duquesne, S., Magnet, S., Jama, C. and Delobel, R., “Thermoplastic Resins for Thin Film Intumescent Coatings: Towards a Better Understanding of Their Effect on Intumescence Efficiency,” Deg. Stab., 88, 63-69 (2005).
- Fream, A., “Hot Prospects for Steel Protection,” Paint Coat. J., 34-37, (Oct. 2004).
- Pimenta, J.T., Gonçalves, C., Hiliou, L., Coelho, Jorge F.J., Magalhães, F.D., “Effect of Binder on Performance of Intumescent Coatings,” Coat. Technol. Res. (Sept. 2015).
- CEPE Guidance to a quality control fire test regime for intumescent coatings, May 2011.
- Sakumoto, Y., et al., “Study on Durability Evaluation of Intumescent Coating for Steel Structures,” J. Mater. Civil Engin., 13, Issue 4 (Aug. 2001).
- Anees, S.M. and Dasari, A., “A Review on the Environmental Durability of Intumescent Coatings for Steels,” Mater. Sci., Vol. 53, No.1, 124-145 (2018).
- Silva, H.M., Cabral-Fonseca, S., and Rodrigues, M.P., “Water Resistance of Intumescent Coatings for Fire Protection of Steel Structures,” LNEC, Portugal.
- UL2431, “UL Standard for Safety Durability of Fire Resistive Coatings,” 2nd Ed., 2014.
- EN 16623: “Paints and Varnishes—Reactive Coatings for Fire Protection of Metallic Substrates—Definitions, Requirements, Characteristics and Marking.”
- ETAG 018, Guideline for European Technical Approval of Fire Protective Products; Part 2: Reactive Coatings for Fire Protection of Steel Elements, EOTA.
- Wang, G., and Yang, J., “Influences of Binder on Fire Protection and Anti-corrosion Properties of Intumescent Fire Resistive Coating for Steel Structure,” Coat. Technol., Vol. 204, 8, 1186-1192 (Jan. 2010).
This article was presented at the American Coatings CONFERENCE, April 9–11, in Indianapolis, IN.
CoatingsTech | Vol. 14, No. 7 | July 2018
The durability and reliability of the fire protection system are very important, because it is essential that the performance of these reactive fire protection systems be maintained under service conditions. The key ingredients of intumescent coatings, APP, PER, and MEL, all exhibit slight solubility in water, so it is perhaps not surprising that durability has become a topic of concern for the industry.10-12
As the fire testing standards such as EN 13381 are concerned only with fire testing, and cover no aspect of durability, new additional standards have been developed and introduced in recent years to cover this aspect, for example, UL243113 for the United States and EN16623/ETAG 01814,15 for Europe.
Durability Standards
$1
United States: The UL2431 standard is intended to provide a means to measure the ability of fire-resistive materials to retain their fire-resistive properties after being subjected to various conditioning environments. The original UL2431 standard was designed for durability testing of spray-applied fire-resistive materials (SFRM–cementitious type fireproofing) but has been revised in the past few years to also cover IFRM (intumescent fire-resistive materials).
The standard defines five classification categories, depending on the exposure type and severity of exposure (see Table 2), to which the fire-resistive material will be subjected during service. The standard then defines the tests to be used for accelerated testing of the coatings for each classification category.
Europe: The EN 16623 standard, first published in 2015 and currently under review, is expected to form the basis for a future harmonized European standard (hEN) for intumescent fire protection coatings for metallic substrates. The ultimate objective is to establish a level playing field for reactive (intumescent) coatings for cellulosic fire situations, but it may take several years before a finalized version appears.
The classification system used in EN 16623 was originally devised as part of the European Technical Assessment system for intumescent (reactive) coatings and is included in ETAG 018. Categories related to exposure conditions within the standard, which is a refinement of those listed in ETAG018-2, are also outlined in Table 2, in comparison with the U.S. categories. The main evolution of the categories between those in the original ETAG 018 and those described in EN 16623 is the addition of the three “W” categories, which cover off-site (factory applied) intumescent coated structural steel components that may be temporarily exposed to the weather for up to six months after delivery to the construction site.
In parallel with the UL2431 standard, each exposure type has its own well-defined accelerated testing regime.
Waterborne Intumescent Coatings
Today, in the cellulosic fire segment of the market [“cellulosic” = fire event fueled by cellulosic materials (paper, card, wood, textiles, etc.) as opposed to “hydrocarbon” = fire event fueled by hydrocarbons
(gasoline, diesel, etc.)], both solvent-based and waterborne intumescent products exist. Although waterborne intumescents have experienced important growth in recent years, especially for on-site application, their use today is still mainly limited to interior environments, because they have a number of well-known weaknesses, not least of which is poor “durability,” related to their high sensitivity to humidity and water.
Additionally, waterborne intumescent coatings tend to have a narrower application window and can be slow drying in thick films, which limits the maximum application thickness per coat. Additional issues sometimes encountered include poor in-can storage stability (especially at low temperature, ca. 5°C), poor freeze-thaw resistance, and occasionally overcoating issues (due to re-solubilization of the intumescent coating by waterborne topcoats).
Current Binder Technology for Waterborne Intumescent Coatings
The vast majority of commercial, waterborne intumescent coatings are based on vinyl acetate (VA) copolymers, most commonly VA/vinyl versatate (VV), VA/ethylene/VV, or VA/acrylic polymers. There is a very good reason for this: VA is relatively low cost and is technically ideal for intumescence, because it is self-charring. The thermal degradation and char forming characteristics of VA are related directly to the chemistry of vinyl acetate copolymers: they contain oxygen, and have a tendency to dehydrate and form double bonds when heated (charring is a chemical process of incomplete combustion of a solid).
PVAc (polyvinylacetate homopolymer) is very water sensitive because the vinyl acetate group is easily hydrolyzed. Because of this, VA is usually copolymerized with other monomers, such as VV or acrylic, which offers steric protection of the vulnerable vinyl acetate group from hydrolysis. Although the water resistance of such copolymers can be improved compared to PVAc homopolymers, it is a fact of life that the higher the proportion of hydrolysis-resistant monomer that is incorporated into the polymer, the better is the water resistance. However, the thermal degradation characteristics are negatively affected and intumescent properties are much reduced.
The high-water sensitivity of current waterborne intumescent coatings can be clearly demonstrated by a simple immersion test in water. After less than half an hour, the coating swells, softens, and blisters, and its intumescent performance is considerably reduced because of loss of water-soluble intumescent ingredients. By comparison, a solventborne intumescent will resist more than five hours, with no blistering or loss of intumescent performance.
This weakness may not appear to be important, since many intumescent coatings are designed for service in dry, interior environments. However, it is important to note that although a reactive (intumescent) coating system may be intended for internal use only, the construction process may result in the coating system being subjected to exposed conditions for a period before the building envelope is closed. In this case, either special provisions need to be made to temporarily protect the exposed reactive coatings, or the reactive coating needs to be evaluated as if it were to be used for exposed applications, (the “W” categories of EN 16623).
New Binder for Waterborne Intumescent Coatings
$1
Seeing no way around the vinyl acetate copolymer performance/water sensitivity issues, we decided to base our new binder on the tried and trusted styrene-acrylic technology that has been the mainstay of solventborne reactive (intumescent) coatings for the past 40 years, and which is well-known for its excellent resistance to water. The advantage of this approach, therefore, is to develop a binder to enable the formulation of durable water-based intumescent coatings.
A new binder (referred to here as HPL211) has been developed specifically for the formulation of waterborne intumescent coatings. It is a styrene-acrylic emulsion copolymer, designed without the use of APEO surfactants and formaldehyde, that fully conforms to the current REACH requirements. The monomer composition has been adjusted to obtain the same good melt characteristics, and synergy with other standard intumescent ingredients (APP/PER/MEL), of the traditional solventborne resins, which results in an excellent and uniform char consistency. Char structure is one of the main parameters of effective insulation. A honeycomb char structure with uniform, dense, and regular cells, without micro-cracks or large voids, provides the best insulation properties.16
Typical physical properties of the new binder are presented in Table 3.
The new binder has been designed to mimic the thermal degradation of our company’s solventborne resins that have been widely used in intumescent coating formulations for many years. Degradation profiles of the new binder and traditional solventborne resin have been compared by thermal gravimetric analysis, as shown in Figure 1. It is clearly evident that the two binders begin to decompose over the same temperature range both for the resins alone and in a blend with solid chlorinated paraffin (CP), which is typically used in solventborne formulations. Moreover, decomposition occurs over the same range of temperature as the decomposition of the standard intumescent ingredients such as PER, MEL and APP, which makes for excellent intumescence and char formation of the coating.
Formulation Approach for New Waterborne Binder
$1
Two formulations have been developed with binder HPL211:
- A “Standard” formulation (referenced as WB IC 01, designed to provide excellent fire protection at competitive cost for EN 16623 durability categories Z1, Z2, and Y, or UL 2431 categories II A-1 to A-3. This formulation uses pentaerythritol (PER) as the carbon source (carbonific). PER has a relatively high-water solubility (5.25% according to the typical value given by the manufacturer, Charmor) but gives optimized char expansion.
- A “Premium” formulation (referenced as WB IC 02) designed to provide excellent water resistance and fire protection for all durability categories [Z1 through X (depending on topcoat used)]. This formulation uses dipentaerythritol (DIPER), which exhibits low water solubility (0.22% according to typical value from the manufacturer) and allows for the optimization of water resistance.
These two formulations, prepared by high speed dispersion, are based on the recipe structures shown in Table 4.
Experimental
Testing Protocol for Evaluation of New Binder
As fire resistance is the most critical test, laboratory evaluation begins with this step, using an indicative evaluation of fire resistance of the coating on a vertical steel plate in a small-scale laboratory furnace. This type of test is on a much smaller scale than the full-scale tests carried out by independent test laboratories for purposes of certification of fire protection products. Nevertheless, small-scale plate tests are commonly used for assessment of primer compatibility and durability tests, because even though small-scale, this kind of evaluation can give a lot of important information on the efficiency of the insulation, char consistency, and char thickness.
Fire Resistance of Intumescent Coatings in a Small-Scale Furnace
Cellulosic fire resistance tests on structural steel, such as those mentioned in Table 1, generally use a standard heating regime that follows the ISO 834 curve. The temperature development of the ISO 834 curve is described by the equation: T=20+345*LOG (8*t+1), which means that the test furnace attains a temperature of around 950°C after 60 min. An uncoated steel section placed in the furnace will gradually heat up, the lag between the furnace temperature and the temperature of the steel being related to the heat capacity, or massiveness of the steel, which is expressed as the section factor (Hp/A m-1). This is the ratio of the exposed perimeter of the steel section to the cross-sectional area of the steel: the more massive the steel section, the lower the Hp/A, the more heat it can absorb, and the longer it takes to attain the “failure” temperature (typically > 500°C). In other words, the higher inherent fire resistance the steel section has, the less fire protection it requires.
When a steel section coated with intumescent is exposed to the same furnace conditions, the steel heats up. Once the coating has intumesced to form a protective insulating layer, the rate at which the temperature of the steel increases is considerably slowed down [seen as an inflection on the temperature/time curve of the steel section (Figure 2)] until eventually it reaches the failure temperature. The time it takes to reach the failure temperature is the fire resistance time of the coating.
For the small-scale furnace tests, coatings are applied at a dry film thickness of 1 mm, usually by brush, to 5 mm thick mild steel panels of dimensions 200 x 300 mm. Two K-thermocouples are attached to bolts welded to the back of the panels, allowing the back panel temperature to be recorded. The furnace is heated by means of two propane burners, under computer control. The heating regime of the furnace is designed to follow the ISO 834 temperature/time curve for cellulosic fire. The insulation ability (fire resistance) of the intumescent coating is assessed as the average time for the two thermocouples to reach 500°C. Thickness and consistency of the resulting char are also recorded.

Durability Test
Accelerated aging tests for evaluation of durability are carried out in accordance with the procedures described in ETAG 018-2 and EN 16623-2015. For durability conditions X and W, painted panels are exposed to UV and water cycles carried out in a Q-UV cabinet following the conditions of EN ISO 16474-3:2013, Table 4, Cycle 2 with a cycle of five hours of exposure to UV-A and 50°C, followed by one hour of water spray.
For Y conditions, painted panels are subjected to accelerated aging comprising temperature and humidity changes as described in Table 5. The test is carried out in a climatic chamber that offers temperature and humidity control over 14 days, thus two times the cycle shown in Table 5.
Results and Discussion
In the first part of the study, the binder HPL211 was compared to a VA-VV latex recommended for intumescent coatings in formulation WB IC 02 at the same binder level. The water soak test and exposure to W and Y conditions were assessed comparatively for the two technologies. In the second part of the study, fire resistance of the formulation WB IC 01, based on HPL211 was compared to commercial intumescent coatings, both solventborne and waterborne systems.
Comparative Evaluation of PHL211 with Commercial VA-VV Latex
Water Soak Resistance
$1
Panels painted with intumescent paints were half-immersed in water over three hours. After drying, coated panels were subjected to heating according to the ISO 834 curve over 10 min.
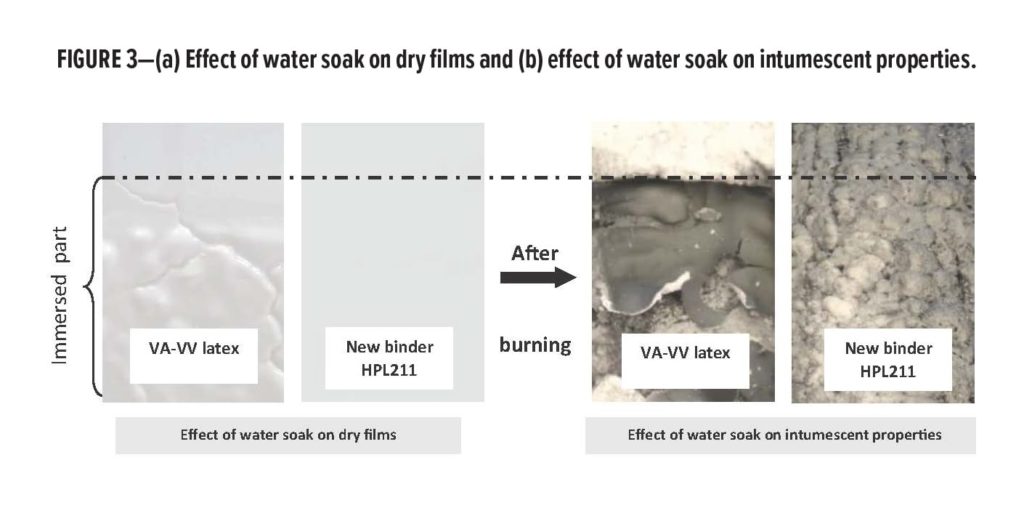
The photos in Figure 3 demonstrate quite clearly the superior water resistance of HPL211 compared to the commercial VA-VV latex as the paint film of the HPL211 formulation was not visually affected by water immersion, while that of the VA-VV latex showed serious blistering and loss of adhesion. Furthermore, the coating based on HPL211 maintained its intumescent properties, while that based on the VA-VV latex no longer showed any expansion on the part that had been immersed.
W Conditions Exposure
Mild steel Q-panels painted with the two paints were exposed to W conditions in Q-UV over five days. Panels were visually assessed after the accelerated aging cycles (Figure 4).

Whereas the coating formulation WB IC 02 based on HPL211 exhibited no defect, the same formulation with VA-VV latex showed cracking and blistering due to water absorption and high-water sensitivity of this binder.
Y Conditions Exposure
$1
Coatings were applied at a dry film thickness of 1 mm on steel panels for assessment in the small-scale furnace. After one week of drying at ambient temperature and one week at 70°C, the panels were submitted to Y conditions cycling over two weeks. After three days recovery at ambient temperature, fire resistance was assessed. Results are presented in Table 6.

Both paints fulfill the requirement for Y conditions since the fire resistance after exposure is within 85% of the fire resistance of the non-exposed panel.
Although formulation with VA-VV latex and pentaerythritol shows normally good intumescence properties and fire resistance, the formulation using dipentaerythritol, which is used for durable coatings, showed a poor quality char with low expansion and large voids.
The formulation with the binder HPL211 gives good fire resistance even after Y exposure conditions, confirming, therefore, the good suitability of this product for durable intumescent coatings.
Comparative Evaluation of HPL211 Formulation with Commercial Products
The formulation WB IC 01 was compared to commercial water- and solventborne intumescent coatings obtained from the European market. Fire resistance was assessed on steel panels coated with 1 mm of intumescent coating. Results are presented in Table 7.
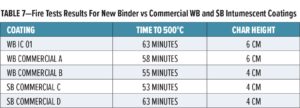
The formulation WB IC 01 based on HPL211 demonstrates very good expansion and fire resistance, similar to, or better than, that of best-in-class intumescent coatings, as shown in Figure 5.
Moreover, the char structure produced by the formulation
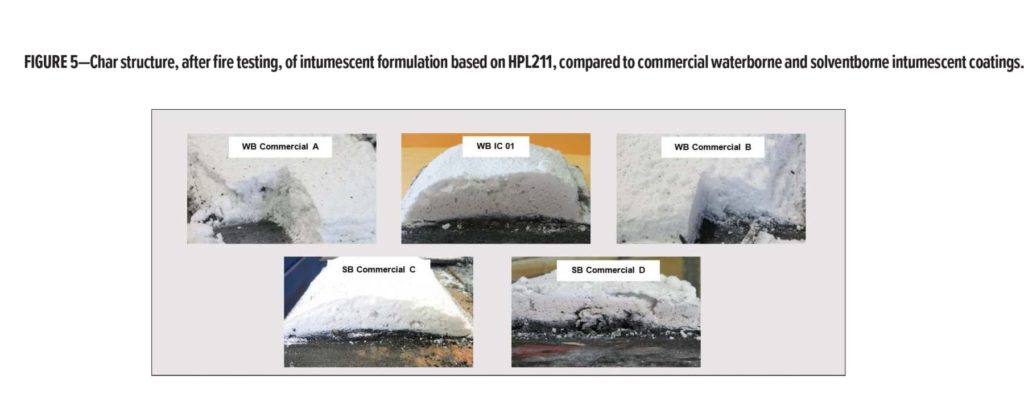
(WB IC 01) is very consistent: uniform with small cells and crispy texture, which is, as discussed previously, the ideal structure for thermal insulation. Despite very good fire resistance and expansion, the chars formed by conventional waterborne intumescent coatings exhibit a softer, more powdery aspect, which is not an ideal consistency as far as “stickability” is concerned, since the environment within a large-scale test furnace as used for fire assessments can present a lot of turbulence.
Further formulation and testing has highlighted the excellent pigment binding capability of the new binder, and the ability to give acceptable fire resistance in the small-scale furnace test at very low binder levels, while still maintaining a firm char structure and good “stickability.” This means that if water resistance is not an important criterion for the product (e.g., for interior exposure conditions), the new binder is capable of giving very economical formulations.
Conclusions
Structural steel design is a vital component of modern architecture. Steel is a versatile building material, and its high load-bearing capacity facilitates structures with wide spans that integrate perfectly into building designs and accentuate specific architectural features. Building regulations dictate that consideration be given to safety aspects of the construction, and that sometimes may appear to conflict with the modern, ornate style of architecture. Intumescent coating systems are perfectly suited to resolving this conflict: through their corrosion and fire protection, they cost-effectively fulfil all the technical requirements and, at the same time, accentuate the aesthetic appearance of a steel structure.
Traditionally, water-based intumescent coatings based on VA polymers or copolymers exhibit good intumescence properties thanks to their thermal degradation behavior, but demonstrate poor water resistance linked to their chemistry.
A new water-based binder has been developed based on the same chemistry and expertise as our company’s well-known solventborne resins for intumescent coatings. This binder can be used to formulate products that have best-in-class fire resistance performance and that meet the demands of the new durability testing standard, like EN 16623 and UL2431.
The superior water resistance of the binder HPL211, compared to current existing water-based technology, has been demonstrated using water soak and durability tests according to ETAG 018/EN 16623 in this study. The better compatibility with intumescent raw materials for durable intumescent coatings has also been evidenced in a typical water-based formulation. The high pigment binding power of the new binder gives the opportunity to formulate very competitive intumescent formulations where high levels of durability are not required.
Acknowledgments
The authors would like to thank Cécile Fonteneau, who carried out the polymerization work for this project.
References
$1
- Grenfell Tower Fire, e.g., https://en.wikipedia.org/wiki/Grenfell_Tower_fire.
- Ahrens, M., “High-Rise Building Fires,” National Fire Protection Association (NFPA), Nov. 2016.
- Morris, P., “Fire Protection of Structural Steel Using Intumescent Coatings,” Fire Safety Professional, Summer 2005.
- Duquesne, S. and Bourbigot, S., in Fire Retardancy of Polymeric Materials, 2nd Ed., Wilkie, C. and Morgan, A. (Eds.), CRC Press, Boca Raton, FL, 254-255, 2009.
- Duquesne, S., Magnet, S., Jama, C. and Delobel, R., ”Intumescent Paints,” Fire Protective Coatings for Metallic Substrates, European Materials Research Society Spring Meeting, Strasbourg, June 10-13, 2003.
- Duquesne, S., Magnet, S., Jama, C. and Delobel, R., “Thermoplastic Resins for Thin Film Intumescent Coatings: Towards a Better Understanding of Their Effect on Intumescence Efficiency,” Deg. Stab., 88, 63-69 (2005).
- Fream, A., “Hot Prospects for Steel Protection,” Paint Coat. J., 34-37, (Oct. 2004).
- Pimenta, J.T., Gonçalves, C., Hiliou, L., Coelho, Jorge F.J., Magalhães, F.D., “Effect of Binder on Performance of Intumescent Coatings,” Coat. Technol. Res. (Sept. 2015).
- CEPE Guidance to a quality control fire test regime for intumescent coatings, May 2011.
- Sakumoto, Y., et al., “Study on Durability Evaluation of Intumescent Coating for Steel Structures,” J. Mater. Civil Engin., 13, Issue 4 (Aug. 2001).
- Anees, S.M. and Dasari, A., “A Review on the Environmental Durability of Intumescent Coatings for Steels,” Mater. Sci., Vol. 53, No.1, 124-145 (2018).
- Silva, H.M., Cabral-Fonseca, S., and Rodrigues, M.P., “Water Resistance of Intumescent Coatings for Fire Protection of Steel Structures,” LNEC, Portugal.
- UL2431, “UL Standard for Safety Durability of Fire Resistive Coatings,” 2nd Ed., 2014.
- EN 16623: “Paints and Varnishes—Reactive Coatings for Fire Protection of Metallic Substrates—Definitions, Requirements, Characteristics and Marking.”
- ETAG 018, Guideline for European Technical Approval of Fire Protective Products; Part 2: Reactive Coatings for Fire Protection of Steel Elements, EOTA.
- Wang, G., and Yang, J., “Influences of Binder on Fire Protection and Anti-corrosion Properties of Intumescent Fire Resistive Coating for Steel Structure,” Coat. Technol., Vol. 204, 8, 1186-1192 (Jan. 2010).
This article was presented at the American Coatings CONFERENCE, April 9–11, in Indianapolis, IN.
CoatingsTech | Vol. 14, No. 7 | July 2018
