By Dr. Douglas Corrigan, The ChemQuest Technology Institute
This article outlines an unmet industry need for formulators to conduct robust testing of their coatings products in real-world or simulated environments that closely resemble an OEM facility’s environmental and processing conditions as coatings are applied and cured. The illustrative examples provided in this article are not hypothetical but are based on actual OEM and end-user processes, widely available coatings products, and current market dynamics.
Application and curing dynamics of paints for factory and field-applied coatings are rarely quantified by coatings practitioners over a wide range of climactic and factory-specific conditions; yet, this information is critical to the long-term performance of the coating in global markets.
For example, there is a growing need to better understand the performance of factory and field-applied coatings exposed to fluctuating humidity and temperature conditions (as shown in Figure 1) during application and curing. For raw material suppliers, formulators, third-party engineers, and applicators, the physics and chemistry of changing climactic conditions during application and curing that impact the durability of a coating system are not well understood. This is primarily due to the disconnect of evaluating coatings performance following application and curing in a highly controlled (small scale) laboratory compared to an evaluation on the scale of an OEM manufacturing environment using similar (if not identical) equipment and techniques.
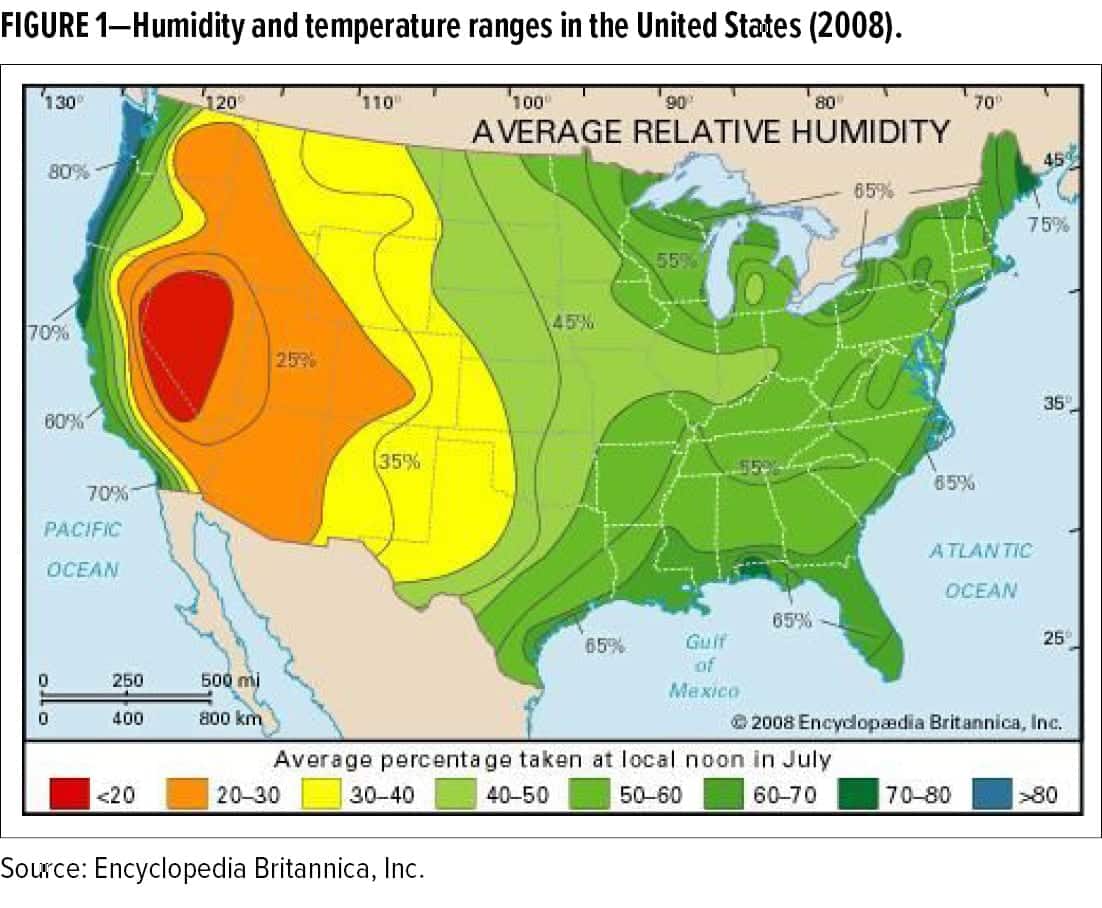
Humidity and temperature conditions affect fluid dynamics associated with the spray stream, the formation and adhesion of the film on the substrate surface, the curing dynamics, and the long-term performance of the cured film. In addition, the physical and chemical variables introduced during fluid delivery process such as pump mechanics, tubing chemistry, spray applicator geometry and mechanics, and air flow dynamics, introduce further complexities in developing a coating for application in the factory or field; worldwide plant locations that use different types of application equipment and methods will clearly add more variables to the evaluation.
We will examine four illustrative examples of how coatings performance test findings rendered in real-world manufacturing environments differ from standard laboratory coatings evaluations:
- Indoor aerospace production shutdown due to weather changes;
- Pretreatment of automotive plastic parts (application window deviations);
- The effects of part geometry on powder coating curing dynamics; and
- Spray dynamics.
Consolidation’s Role
Historically, smaller and mid-sized paint manufacturers provided custom products and exceptional service by today’s standards to small manufacturers of parts for OEM assemblies, often including a specific paint SKU for a part. Coatings were tested through plant
trials on a part manufacturer’s production line, usually with the coatings supplier on-site at their customer’s location to observe the testing.
If the test results were negative—no matter how small or large of a correction might be required—the mid-size formulator had the capacity, knowledge, experience, and resources to make changes to the formulation or to recommend changes to a customer’s process. The coating formulator’s recommendations were usually tailored to the plant’s specific environmental conditions.
In recent years, inorganic growth (consolidation) has shifted priorities for newly reorganized paint and coatings manufacturers following an acquisition or merger: the exceptional technical service on which smaller (low volume) OEM customers depended, in many cases, was no longer available. A void emerged in the standard protocol for testing and selecting a coating for an OEM part, especially for testing new coatings products. The responsibility for testing a coating for its intended application or use shifted away from the coatings formulator to the part manufacturer. The low-volume part manufacturer had little choice but to adapt by selecting a non-custom (off the shelf) coating product that is not optimized for its intended application, or to switch to toll manufactured coatings.
By comparison, large OEMs with high-volume production continued to receive individualized technical service from multinational coatings formulators. Yet as paint companies grow and begin serving a larger customer-base in new geographic regions (moving away from the smaller regional player’s business model), they are faced with having to produce coatings systems to meet global performance standards. Will a coating originally formulated for use in a midwestern automotive plant in the United States, that is tested in a controlled laboratory, perform as well in Thailand?
The responsibility is squarely on the formulator to test their products over wider environmental ranges, and to formulate their coatings to be more robust to handle those varying conditions. Yet the standard array of lab tests either entirely ignores relative humidity (RH) and temperature (T)—or tests at a single value—when, in fact, a wide set of values is in order.
The solution may involve designing multiple formulas and SKUs for different conditions (i.e., one formula optimized for hot and high humidity climates; another formula for cold temperatures and low humidity). However, pricing pressures and other factors mitigate against that type of compromise.
Alternatively, developing one formula that is not optimized for a single environment, but instead is designed to perform adequately in multiple conditions and environments may be less costly and acceptable for certain end uses. These compromises ultimately result in performance disadvantages. Making formulation decisions to optimize this balance between performance and widening the application window is predicated on being able to develop reliable test data under real-world conditions.
Aerospace Production Shuts Down When Weather Changes
In this scenario, an aerospace manufacturing facility specified a coating with a technical data sheet (TDS) that expressly stated coatings performance could not be guaranteed in application conditions of 40% or less humidity. Consequently, when the facility’s indoor RH dropped below 40% due to outdoor weather conditions, the facility’s painting operation was halted until the indoor RH increased, causing a large loss in production output and associated revenue. The risk of liability associated with applying a coatings system in low humidity conditions that would not be certified by the coatings manufacturer was too great. At issue for the end user was the absence of application testing data for verification purposes that would ensure that the coating would perform at the lower RH levels in their factory using the same type of spray equipment, curing process, etc.
The TDS stipulated < 40% RH as the lowest criteria simply due to a lack of adequate application data below this level. The coatings user did not have the ability to conduct the necessary testing given that the conditions inside their factory depend on difficult-to-forecast weather. Therefore, planning a plant shutdown on a low humidity day was not realistic.
However, if the paint manufacturer could test the paint in a simulated manufacturing environment under those low humidity conditions—and assuming the test results met with FAA approval and exhibited overall satisfactory performance—they could confidently modify their TDS based on the new test data. This outcome would pave the way for expanded usability of the coatings manufacturer’s product, resulting in higher sales, increased market penetration, potentially leading to new market opportunities. The end user, in turn, could manufacture for a larger percentage of the year, and achieve higher throughput and profitability.
By comparison, conducting coatings tests on the scale of a laboratory in a small test chamber using standard lab application equipment and curing methods would not render the same findings as a low humidity simulated application and manufacturing environment. This is mostly because laboratory testing assesses temperature and humidity effects only during curing; neither temperature nor humidity is measured during application. The actual testing would need to closely mimic every aspect of the aerospace painting operation, including multiple low humidity values of 10%, 20%, 30%, and 40%—
factoring in the actual substrate types, the spray procedure and equipment (same type of pumps and spray guns), and the same curing method, followed by relevant ASTM tests in plant conditions.
Pretreatment of Automotive Plastic Parts (Application Window Deviations)
Plastic automotive components normally undergo certain surface pretreatment processes prior to application of coatings, adhesives, and foams. These processes are used on low surface energy materials to clean and activate the surface to improve wettability and adhesion. Among other factors, the timing between those two processes will ensure the quality and performance of the finished part. To achieve the required wettability and adhesion properties for this application, a particular automotive tier supplier opted to raise the free surface energy of the plastic substrate by using an open-air plasma pretreatment process.
Conducting tests following pretreatment is a common procedure for assessing to what degree, if any, factory conditions will affect the application window. This assessment is necessary because any type of pretreatment will render an exposed surface vulnerable to local conditions of temperature and humidity prior to applying coatings.
Under typical factory conditions, an approved coating process may include an acceptable window of time between the pretreatment and coating application. For illustrative purposes, we will use a 15-min application window as the norm. Unbeknownst to the tier supplier, that standard 15-min application window may one day shrink to five minutes. This sudden deviation in the application window can be caused by high or low humidity plant conditions, or other factors—which, in turn, can affect the part’s surface energy following pretreatment. The effect of low or high humidity on the substrate performance is not normally known because it may have never been tested, nor is it addressed in the plant’s coatings specification or in the supplier’s TDS.
If these parameters are not established in advance, the factory workers will allow the parts to proceed through the manufacturing process. Therefore, the prepared surface following pretreatment may be left exposed to the current high or low humidity condition for more than five minutes. Consequently, deviations in adsorbed moisture caused by the interaction between the gas phase and the solid phase of the substrate, the temperature of the part, and the dew point of the air, will create a surface condition that may not be amenable to proper coating or adhesive performance. As a case in point, Figure 2 demonstrates the effects of T and RH on the free surface energy of an exposed low surface energy plastic membrane over time after pretreatment with an open-air plasma treatment process.
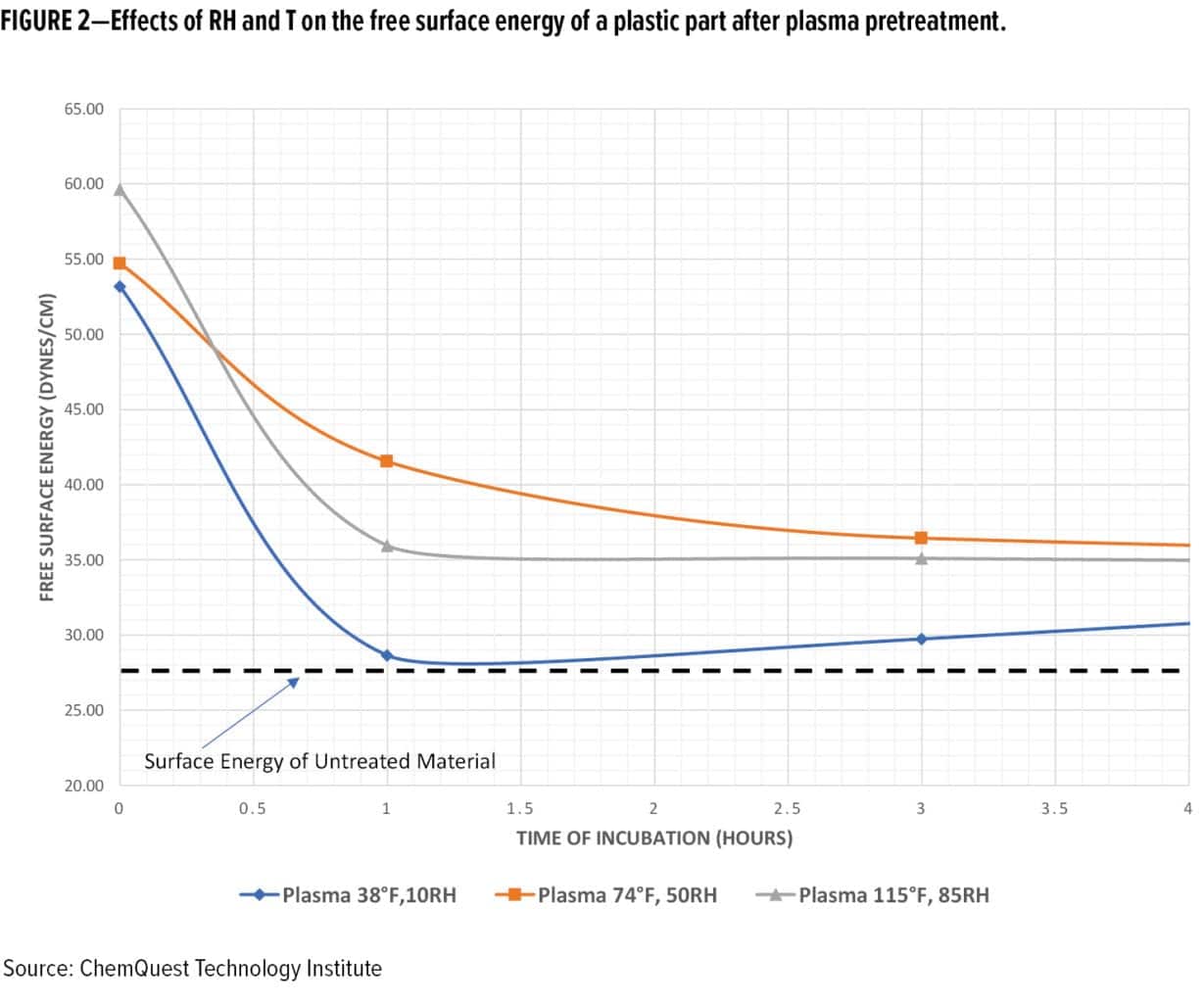
Dew point can affect the performance of field-applied and factory-applied coatings, especially in large factories with indoor air conditions that change with the weather. The drop of water on the surface of a pretreated part may be at the atomic or molecular level often not visible to the human eye, unless the part begins to “sweat.” Even a molecular layer of water between the primer and the substrate can interfere with the adhesion of the primer to the substrate. Because of these interrelationships, control of the humidity and temperature during the application process is important to realizing a cured film that has superior physical and chemical performance attributes. In the absence of controls, premature failure of the coatings system may occur as follows:
- Reduced corrosion resistance;
- Changes to free energy of the surface changes to film formation;
- Blistering, blushing, cracking, delaminating, and blooming defects;
- Loss of adhesion; and
- Degraded chemical resistance.
It is best practice to ensure that the temperature of the substrate is at least 3–5°F above the dew point of the air. Preventative measures when that condition is not met might include raising the temperature of the part to avoid condensation onto its surface prior to painting. However, this measure is impractical in the field, and costly in the factory because it is an added process.
In summary, there is a measurable advantage in a part manufacturer’s process flow to leveraging test findings under a variety of factory conditions.
Effects of Part Geometry On Powder Coating Curing Dynamics
Curing a powder coated part typically involves placing the part in an oven set at a certain temperature and allowing the part to cure for a set time. For example, a powder coating may call for a curing temperature and time of 400°F for 10 min. Most OEMs have ovens that comfortably reach the desired temperature set point. However, what is not normally considered in product and process development is the myriad of sizes, shapes, and mass of parts—all of which affect the ultimate thermal profile that the coating will experience during its trek through the oven. Also, the type of oven and air flow through the oven will affect the thermal profile.
In this scenario, following a supplier’s instructions in the TDS, a particular OEM proceeds to powder coat its part by placing it in a 400°F convection oven. After 10 min, the part is removed from the oven and a visual inspection indicates it is properly cured (no visual defects). Next, the OEM begins conducting standard tests on the part’s coating; tests that may include a mandrel bend test, methyl ethyl ketone (MEK) solvent rub test, followed by more standard test methods for coating adhesion and hardness. One-by-one the coated part fails these tests. Why? What went wrong? Is the chemistry or production process of the powder coating responsible, or is the failure due to differences in thermal mass of the parts, and the temperature profile of the OEMs oven?
An investigation reveals the large thermal mass (e.g., a block of steel) of the part prevented it from reaching 400°F (even though the convection oven did) because a part with a large thermal mass does not heat as quickly as a smaller part. This variation is a function of the geometry of the part—10 min is insufficient to heat this larger part to 400°F.
Simply put, the TDS instructions to heat the part for 10 min in a 400°F oven omitted heating variations for different size parts. The TDS also excluded variations in equipment. Ovens vary tremendously: there are different types of ovens (convection is one type), air flow, and methods of heating (gas, electric, and infrared). The formulator oven cured a powder coated standard grade of metal testing panel that is approximately 2 in. x 4 in., with a thickness of 1/16 in. to 1/8 in. With a low thermal mass, 10 min sufficed to cure the test panel in a 400°F oven, which was the basis for the formulator’s TDS curing criteria. However, the OEM’s large thermal mass part did not meet that criteria.
A conflict between the OEM and powder coating supplier might have been avoided if the supplier would have conducted curing tests on a dozen different sized parts, using various types of ovens. Also, the TDS should have spelled out at least the most commonly encountered variables for a formulator’s customer base.
In summary, a process may technically follow the set point and dwell-time parameters as called for in the TDS, but the surface of the part may still experience an insufficient thermal profile, depending on its residence time, geometry, and thermal mass. Testing cure time for a variety of part configurations (different shapes and thermal masses) using different types of ovens is necessary for quality assurance and is critical for accurately and efficiently troubleshooting a coatings failure for both the coating manufacturer and end user. Being proactive, a formulator may ask customers for examples of what they are coating for possible inclusion in the supplier’s test matrix. The intent is to identify a range of testing samples—the highs and lows that represent the extremes of the process—with the understanding of where every sample in between would fit on the spectrum for a TDS.
These findings are not typically included in standard lab tests, so switching to a simulated manufacturing environment for performance testing of powder coated parts makes good business sense.
Spray Dynamics
Droplet Dynamics
Inherent to all spray technologies, whether they are conventional, HVLP, airless, or air-assisted-airless, is the atomization/aerosolization of the bulk liquid into small microdroplets that are moving at relatively high velocity. The fineness and consistency of the atomized droplets are critical factors in the wetting of the surface and the formation of a uniform, continuous film. If the droplet morphology is not consistent and within the proper range upon impact with the substrate, a film may fail to form properly. If the droplet morphology is such that the film builds too quickly, running, sags, and orange peel can result, as well as extreme variations in dry film thickness that, when cured, will result in heterogenous microstructural variations in the physical performance of the film.
As the drop leaves the spray nozzle, it enters an atmosphere of either stagnant or moving air (see Figure 3). This body of air is represented by three parameters:
- Air velocity;
- Temperature; and
- Relative humidity
The dynamics of the drop as it moves through the body of air are characterized by several key parameters, including (but not limited to):
- Drop size (radius)
- Drop temperature
- Viscosity
- Surface tension
- Drop velocity
- Rate of evaporation at surface
- Heat conduction and convection inside the drop
- Water content
- Solids content
- Solvent content
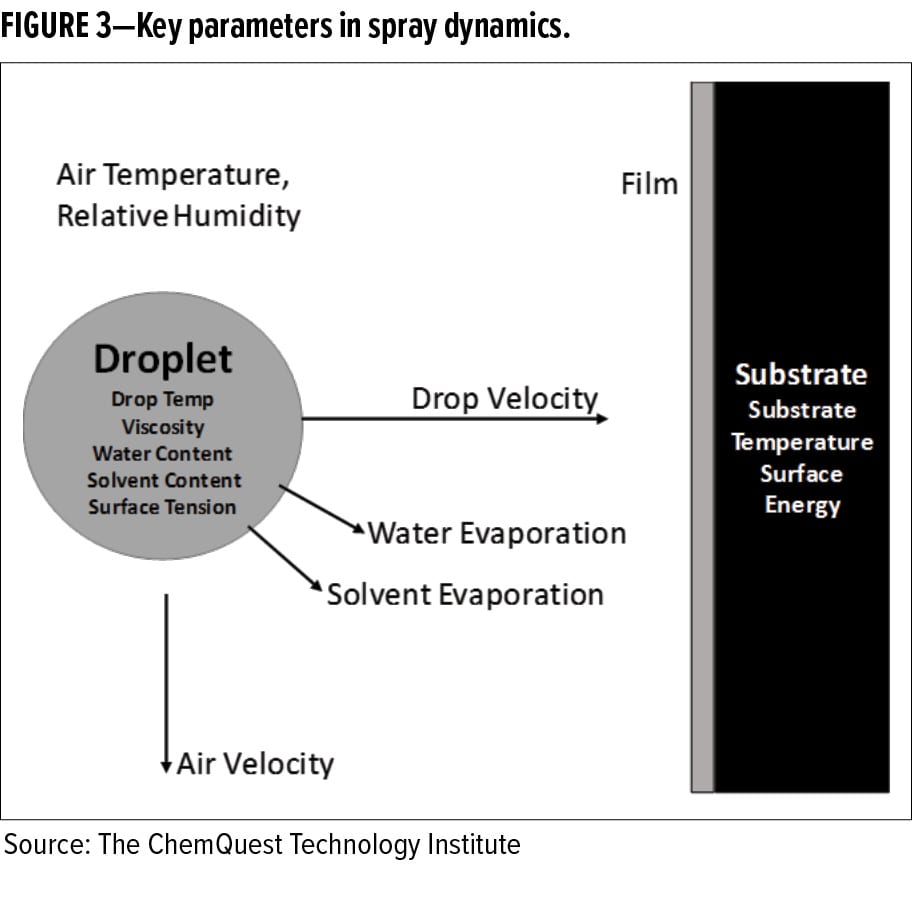
As the drop moves through the body of surrounding air, each of these parameters affects how the droplet evolves as it moves from the spray nozzle to the substrate. For example, a droplet with a relatively high-water content traveling through a body of air that is at relatively high temperature and low relative humidity will lose its liquid content due to rapid evaporation from the drop. The drop will cool due to evaporative cooling, raising the viscosity of the liquid. As the drop travels toward the substrate, it will continue to decrease in size while the solids content increases. If the droplet’s water content is too low at the time it makes impact with the surface, wetting and film formation may fail to occur properly.
Many water-based paints are designed with coalescing solvents and rheology reagents to finetune both the viscosity and the minimum film forming temperature of the coating. The ratio of water to these coatings additives, as well as the relative rate of evaporation of the water and coalescing agents, determine the overall quality of the final film. The quality of film will dictate the efficacy of the paint post-cure. Therefore, from this one simple thought experiment, it is evident how the RH of the environment can affect the final performance profile of the coating.
In the scenario in which the RH is high and the T is lower, the rate of water accretion will be greater than the rate of evaporation, and the droplet will grow as it picks up water from the surrounding air. This phenomenon can cause the paint to have a water content that is too high, which will affect its viscosity. This, in turn, can lead to loss of film build, increased sagging, and increased curing times. If the downstream curing process does not evaporate this accumulated water, this can introduce significant problems in the performance of the film downstream.
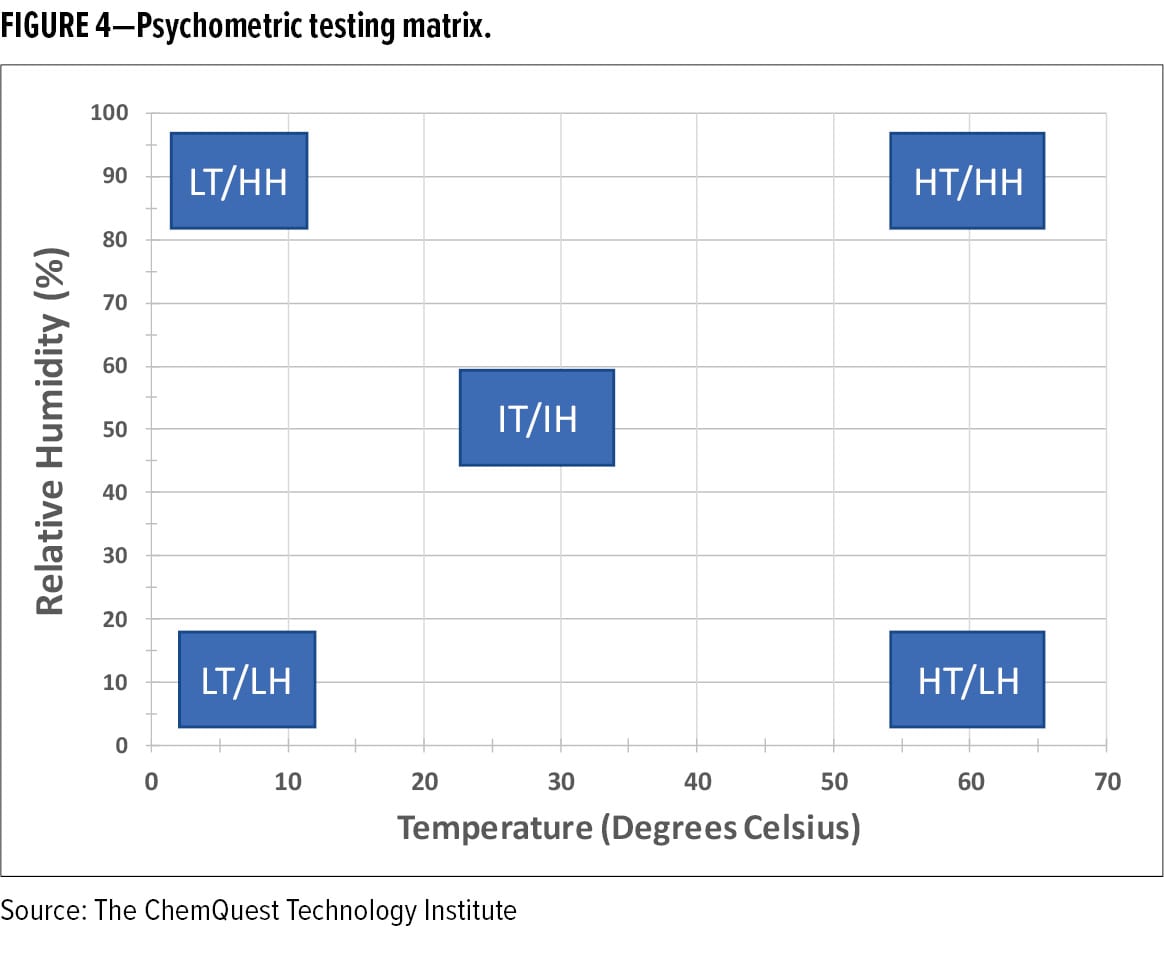
Due to this linkage between environmental conditions, spray dynamics, film formation, and final film performance, it is advantageous to test the spray application of coatings under varying humidity and temperature conditions. Spray testing under five psychometric conditions (low T/low RH; low T/high RH; high T/low RH; high T/high RH; and Intermediate T/Intermediate RH) is a good place to start (see Figure 4). Because temperature and relative humidity are inversely related, achieving low T/low RH and high T/high RH application conditions is a challenge and requires specialized application equipment that is designed to treat and replenish moving air as it becomes laden with solvent and/or water during the application process.
Transfer Efficiency Dynamics
OEMs routinely design application equipment and processes to optimize transfer efficiency and reduce waste. This not only reduces the cost per part due to reduced usage of coating, it also reduces the costs associated with clean up, hazardous waste removal, equipment repair and maintenance, and VOC permitting. Uncontrolled overspray may be removed through a ventilation system or it may adhere to the conveyor or walls of the process equipment. Air pressure, gun-to-part-distance, coatings chemistry and the design, size and settings of the spray nozzle (and even the amount of air in a spray booth) will influence the degree of overspray. Furthermore, transfer efficiency processes are engineered in the plant for recapturing overspray for reuse to reduce waste. One method involves employing squeegees to scrape the liquid coating overspray off the underside of a conveyor belt into a steel container for filtering and reuse.
While paint manufacturers do conduct lab tests to ensure performance they generally are not factoring in their customers’ transfer efficiency methods and sensitivity to these paint characteristics. By testing a formulation in a real or simulated manufacturing environment, a formulation can be optimized to exhibit a higher transfer efficiency and a greater recoverability.
Conclusion
There is a growing need across the paints and coatings value chain to fully understand and plan for a wide range
of variables encountered in field- and factory-applied coatings—variables that are not routinely accounted for in the typical battery of lab tests. Among these variables, temperature and humidity play a crucial role in coatings performance.
Heretofore, data specific to a plant or application has been lacking as well as the technological resources required to conduct spray application trials under user-defined, controlled, environmental conditions. Making a case for proactively obtaining and using this data on behalf of coatings end users would benefit coatings formulators and the value chain in various ways, such as:
Providing needed information to raw material suppliers for the design of new chemistries (resins, solvents, additives, etc.) that operate over a larger range of climactic conditions;
Informing formulators on how to optimize their products for use across disparate climates;
Conveying to end users the best conditions under which to apply coatings and identifying steps they can take to mitigate against defects;
Creating a body of knowledge for writing new standards that correlate product chemistry to best practices for application conditions.
References
Funke, W., “Blistering of Paint Films and Filiform Corrosion,” Prog. Org. Coat., 9 (1) 29-46 (1981).
Erbil, H.Y., “Evaporation of Pure Liquid Sessile and Spherical Suspended Drops: A Review,” Adv. Colloid Interf. Sci., 170 (1) 67-86 (2012).
Voué, M., et al., “Dynamics of Spreading of Liquid Microdroplets on Substrates of Increasing Surface Energies,” Langmuir, 14 (20) 5951-5958 (1998).
Steward, P.A., Hearn, J., and Wilkinson, M.C., “An Overview of Polymer Latex Film Formation and Properties,” Adv. Colloid Interf. Sci., 86 (3) 195-267 (2000).
Nguyen, T., Bentz, D., and Byrd, E., “A Study of Water at the Organic Coating/Substrate interface.” J. Coat. Technol., 66, 39-39 (1994).
