By Ravi Ravichandran, Michael Emmet, Matthew Gadman, John Florio, and Steven Woltornist, King Industries, Inc.
A new class of metal-free catalysts has been developed that promotes the crosslinking reaction of epoxy functional polymers with carboxyl functional compounds and polymers. Particularly effective at much lower cure temperatures, these catalysts provide stable single package formulations and improved resistance properties. In addition, unlike amine-based compounds, these catalysts do not yellow during cure or on over-bake.
INTRODUCTION
Epoxy resins are commercially and technologically relevant and play a critical role in several key applications such as coatings, adhesives, laminates, castings, encapsulations, and moldings. Epoxy-based polymer resins are an important part of the automotive clearcoat and powder coating market segments. Their versatility of crosslinking with various agents [e.g. acids, anhydrides, dicyandiamide (DICY), and phenolics], in addition to their ability to provide superior chemical resistance, extraordinary acid etch resistance, and good adhesion, make epoxy functional resins attractive in several end-use markets.
Epoxy Acid Automotive Clearcoats
During the late 1980s, field damages caused by environmental etch became a major issue especially at automotive import storage areas in Jacksonville, FL. This phenomenon was a clearcoat appearance issue associated with the formation of what appeared to be permanent water spotting. The physical damage caused by etch results in localized loss of material, which in turn leads to visible pitting of the clearcoat surface. The etch phenomenon was believed to be primarily the result of acid-catalyzed hydrolysis of ether crosslinks in acrylic melamine clearcoats due to a combination of acid rain exposure and high temperatures. The resulting crosslink scission on the surface can be a localized phenomenon, leading to defects on the surface that resemble water spotting.
Water spotting damage is irreversible as the chemical degradation of the clearcoat results in increased surface roughness, leading to poor appearance properties, such as reduced gloss. It has since been established that acidic airborne pollutants like SO2 produced in heavy industrial areas are converted to acid rain. The typical pH of acid rain encountered in aggressive environments (Jacksonville, FL) is in the range of 3.5–4.5.1,2 These phenomena have led to the development of etch-resistant clearcoats using new crosslinking chemistries. For example, modifications of acrylic melamine clearcoats made with additional crosslinking with either blocked isocyanates or silane led to improved etch resistance.3 During the thermal cure the alkoxysilane pendant groups undergo aqueous hydrolysis to form silanols, which in turn co-react to form siloxane crosslinks, resulting in enhanced acid-etch resistance. Carbamate functional acrylics crosslinked with melamine resins result in a hydrolysis-resistant urethane crosslink without the use of two-component (2K) polyisocyanate formulations. To achieve the best combination of environmental etch, weathering and scratch resistance, and appearance properties, clearcoats use a combination of crosslinking mechanisms together with acrylic melamines. Isocyanates can be functionalized with alkoxysilyl compounds leading to both urethane and siloxane crosslinks in the coating.3 Similarly, resins with alkoxysilane functionality can also be combined with carbamate-functional acrylics.4
Epoxy-functional acrylics crosslinked with carboxylic acids or anhydrides provide excellent environmental etch resistance. The technology using epoxy/acid crosslinking reaction, very popular with the Asian automakers, is the most robust of them all, producing very powerful acid etch and scratch-resistant coatings, and can be formulated as either one-component (1K) or 2K coatings
The global automotive coatings market size is projected to reach US $16.24B by 2021, registering a CAGR of 7.02% between 2016 and 2021, and witnessing huge growth in emerging economies. Approximately 36% of the global automotive coatings market is in each Asia Pacific and Europe with the remainder in the Americas.5 Emerging economies such as China, India, and Brazil are the key markets for automotive coatings. Emerging middle-class population, changing life style, growing purchasing power parity, and improving standard of living of consumers in these economies are the key factors that drive the demand for automotive coatings. Among all the regions in the world, Asia-Pacific is the largest market for automotive coatings owing to the huge base of automotive industries in that region. It is also the fastest growing market during the period 2014–2020 due to the technological advancement and large number of vehicles produced. It is poised to grow at a CAGR of more than 7% for the next six years.
More than 80% of the clearcoat markets in Japan and Korea are 1K etch-resistant coatings. One-component epoxy/acid is the leading etch-resistant clearcoat technology used by several auto manufacturers, including Nissan, Toyota, and Mitsubishi.
Epoxy Acid-Based Powder Coatings
Powder coatings have become an attractive alternative to conventional coating techniques. This is primarily due to their high efficiency, the durability of resulting finishes, and very low environmental impact. In addition, powder coatings effectively resist chipping, scratching, and fading, leading to high quality finishes that are color-stable. Since no solvents are involved in the process, the powder coatings can be recycled and reused, thereby reducing the emissions of volatile organic compounds (VOCs) and waste generated from the conventional liquid coatings. Powder technology finds widespread use in automotive parts and bodies, architectural, furniture, and many household appliances.
Four major crosslinking chemistries are widely used in thermal powder coating systems. Esterification reaction (acid/hydroxyl reaction) exemplified by hydroxyalkyl amide (Primid®) polyesters, is the least reactive with a cure profile of 150°C–220°C. Hydroxyl/blocked isocyanate reaction leading to polyurethanes, with either blocked isocyanates or uretdiones (isocyanate dimer) can lead to a slightly lower temperature cure with minimum cure temperatures in the 140°C range. The other two chemistries are both based on epoxies that can crosslink with a variety of functional groups including acids, anhydrides, aromatic hydroxyls, amines, DICY, and even through homopolymerizations. Epoxy/acid or epoxy/anhydride has the highest potential for low-temperature cure in the range of 120°C to 140°C, followed by epoxy/phenolic or epoxy/DICY, which are both amenable to cure temperatures as low as 110°C. Higher curing temperatures in the 150–200°C range are not useful with substrates such as plastics, wood, and certain metal alloys, and are limited to substrates that can tolerate higher temperatures. Epoxy homopolymerizations and epoxy/phenolic reactions, while providing lower temperature cure options, lack the UV resistance necessary for exterior applications. Thus, to meet both lower temperature cure potential and adequate exterior durability, formulations based on acid-functional polyesters and epoxy crosslinkers offer the most promise. Polyester epoxy hybrids offer more versatility compared to 100% epoxy, including better external weatherability at a lower cost, and, as a result, have captured a larger share of the market. Polyester-epoxy hybrids and polyester-triglycidylisocyanurate (TGIC) powders are the two main types used in the North American market; together they represent nearly 60% of the market, followed by polyurethanes, epoxies, and acrylics.
Catalysts are used in epoxy powder coatings: (1) to catalyze the reaction of glycidyl ester functional acrylic resins with dicarboxylic acids or with carboxyl functional resins, (2) for crosslinking of carboxyl functional polyester resins with TGIC, and (3) for hybrid powder coatings of bisphenol A diglycidyl resins with carboxyl-functional polyesters.
A catalyst that can lower cure temperatures of epoxy/acid powder systems to 120°C or below would be of interest in powder applications and could result in energy savings and/or higher throughput in industrial coating lines. The advent of low-curing temperature systems will have a significant impact on the powder coatings market utilizing heat-sensitive substrates such as wood, plastics, and assembled components with heat-sensitive details. The coating of metal substrates also benefits from this technology with lower energy and investment costs, shorter curing times, and higher lines speeds.6
Global demand on powder coatings was valued at roughly US $5.8B in 2010 to US $9.1B in 2013, a compound annual growth rate of 7% and is expected to grow by 6% in the coming years.7,8 The powder coatings market is projected to reach US $16.55B by 2024, at a CAGR of 6.75% from 2017 to 2022. Increasing use of powder coatings for aluminum extrusion used in windows, doorframes, building facades, kitchen, bathroom, and electrical fixtures will fuel industry expansion. Rising construction spending in various countries including China, the United States, Mexico, Qatar, UAE, India, Vietnam, and Singapore will fuel growth over the forecast period. Major factors that are driving the market’s growth are the increasing usage of powder coatings in the automotive industry across applications such as door handles, rims, and under-the-hood components; and growing demand for consumer goods in Asia-Pacific, including washing machines, freezer cabinets, and microwave ovens. Industrial uses present the largest application market, with furniture and appliance markets showing steady and strong growth. Information technology and telecom are new markets where applications of powder coatings are developing.
Epoxy-polyester hybrid powder coatings will witness the fastest volume growth at a CAGR of 8.1% from 2016 to 2024 because of their increasing application in domestic appliances, machines, equipment, decorative devices, and general industries. Furthermore, superior properties including greater resistance to yellowing during cure; better transfer efficiency; and cleaner, brighter colors than many epoxy formulations are expected to stimulate industry growth. The epoxy polyester hybrid segment is expected to account for more than 50% of the powder coating market share by 2024.
Binder, Crosslinking Chemistry, and Catalysis
The crosslinking chemistry involves the reaction of an epoxy-functional resin with a hardener containing a carboxylic group, in a ring opening condensation reaction, resulting in stable linkages with excellent chemical resistance properties, and without the formation of any reactive volatiles (Figure 1).
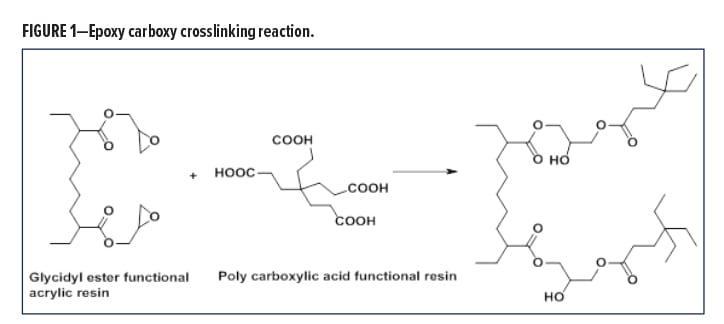
The ester linkage formed is significantly more stable to acid etch and leads to one-package coatings with good storage stability, which can also meet the aggressive artificial acid etch test of Japanese auto producers, like the Toyota Zero Etch Test.
For outdoor applications requiring good weatherability, such as automotive clearcoats, aromatic epoxy resins such as bisphenol A diglycidylether cannot be used, while copolymers of glycidyl acrylates or methacrylates are typically used. The co-monomers are chosen to provide other critical performance properties such as hardness, flexibility, and the like. The structure of the acidic resin is also critical to achieve the desired hardness and scratch/mar resistance properties. A high scratch resistance can be achieved by a combination of high crosslink density and flexible chains between network points, using flexible acidic polyesters as the acid component.9
The hydroxyl group generated during the reaction can be utilized for additional crosslinking reactions with auxiliary binders such as blocked polyisocyanates or polymeric siloxanes containing hydrolyzable silyl group. Such hybrid technologies can provide clearcoats with excellent acid etch and mar resistance.10
Catalysis of the epoxy/carboxyl reaction has been investigated thoroughly in an earlier study, which resulted in the development of a zinc chelate catalyst.11 A wide range of catalysts have been developed for this reaction, which fall into four major classes (Table 1).
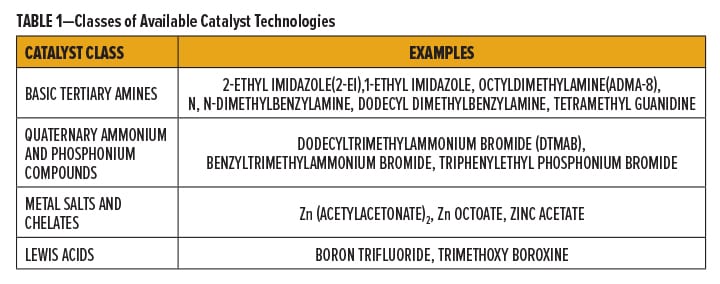
Basic catalysts such as tertiary amines provide high reactivity at lower temperatures, but formulations containing these catalysts suffer from severe yellowing and limited stability, especially in a 1K system. Thus, their volatility, odor, yellowing, and potential hazard labeling make these catalysts unsuitable especially in applications that are sensitive to discoloration.
Quaternary ammonium and phosphonium bromide salts are effective 1K catalysts, with less thermal yellowing on overbake compared to the free tertiary amines. However, their strong basicity can lead to problems with water permeability and humidity resistance. In addition, these catalysts do not impart adequate mar resistance on catalyzed coatings.
Metal salts and chelates are stable at higher temperatures and impart little or no yellowing during cure and through the life of the coatings. Unfortunately, all the state-of-the-art zinc catalysts, while efficient at 140°C, are not effective at lower temperatures, especially at 120°C or below. Although zinc carboxylates are effective catalysts for the epoxy/carboxyl reaction, the divalent zinc can contribute to ionic crosslinking, which leads to storage instability, viscosity increase, and gelation.
Lewis acids are very effective for epoxy homopolymerization but can lead to many side reactions in epoxy/acid cure.12 Super acids, such as trifilic acid, can catalyze the homopolymerization and copolymerization of epoxy resins with hydroxyl functional polymers, cyclic ester, oxetane, and vinyl ether reactants. Most of the cationic catalysts are activated by UV radiation.
Recently, quaternary ammonium blocked hexafluoroantimonate and triflic acid catalysts have been introduced for thermal cationic cure.13 These catalysts function by a rearrangement of the quaternary compound to a weak, basic amine. Lewis and super acids are not commonly used as catalysts in epoxy/acid coating systems.
We began our research towards a more efficient and versatile alternate to the above catalyst options that can provide efficient cure at 120°C, or lower, with good viscosity stability, while imparting no yellowing or discoloration following overbake.
Herein we describe the development of a new class of metal-free catalysts useful in a variety of epoxy/acid systems that are efficient and would lead to the desired lower temperature cure required both in automotive and powder coating applications.
EXPERIMENTAL
The three experiments discussed in the following sections include a series of tests that demonstrate the catalyst capabilities of two new metal-free catalysts for the reaction of carboxyl and epoxy functional resins.
Experiment I investigates the overall efficacy of NACURE® XC-324 as a catalyst in a 1K epoxy/carboxy clearcoat. Experiment II explores lower temperature cure capabilities of NACURE XC-355 in a 2K epoxy/carboxy clearcoat. Studies on environmental etch resistance of epoxy/carboxy clearcoats compared to aminoplast crosslinked polyols are included in Experiment III.
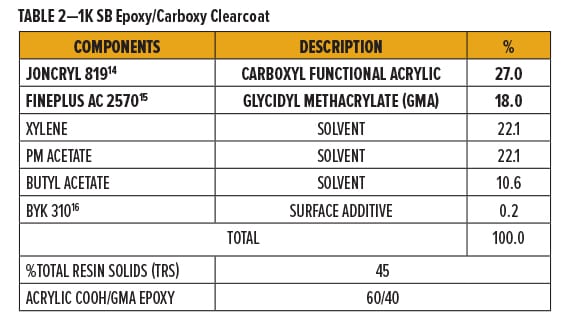
All epoxy/carboxy test formulations in these experiments use commercially available, solid grade resins dissolved in solvent. Solventborne (SB) clearcoats were formulated using a carboxyl (COOH) functional acrylic resin with glycidyl methacrylate (GMA) resins.
Experiment I: Catalyst Studies on NACURE XC-324 in a 1K SB Epoxy/Carboxy Clearcoat
NACURE XC-324 was evaluated in a 1K SB epoxy/carboxy clearcoat against two widely used conventional basic amine catalysts [octyldimethylamine (ADMA-8) and 2-ethylimidazole (2-EI)].
Materials and Preparation of 1K SB Epoxy/Carboxy Clearcoat Formulations
Joncryl 819,14 a carboxyl functional acrylic resin, and Fineplus AC 2570,15 a glycidyl methacrylate resin, were blended at an approximately 1:1 molar ratio and dissolved with xylene, PM acetate, n-butyl acetate, and BYK 31016 to form a 45% resin solution. A breakdown of formulation components is in Table 2.
NACURE XC-324 and the two control catalysts were all post-added to the clearcoat formulation and mixed via high speed dispersion using a FlackTek high speed mixer from FlackTek Inc.
Clearcoats were catalyzed with 1.0% catalyst solids on total resin solids (TRS).
Film Preparation of 1K SB Epoxy/Carboxy Clearcoats
All films prepared for testing were applied via draw down. Epoxy/carboxy clearcoats were applied using a drawn down bar. White waterborne (WB) basecoats used in color testing were applied using a bird applicator. Epoxy/carboxy clearcoats and WB basecoats were both given an approximately 10–15 min flash at ambient temperatures prior to baking at the designated temperatures.
Film Properties of Catalyzed Clearcoats
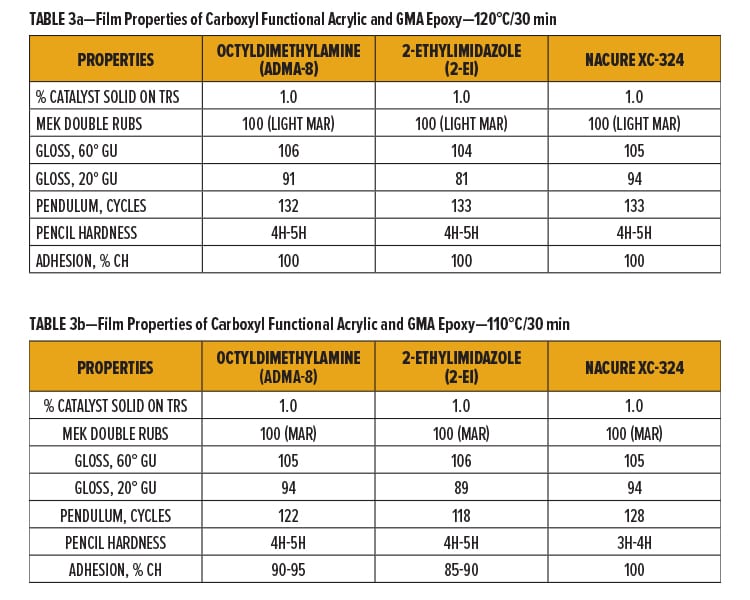
NACURE XC-324 was compared to the control catalysts in the 1K SB epoxy/carboxy clearcoat using two bake schedules: 120°C/30 min and 110°C/30 min. Catalyzed epoxy/carboxy clearcoats were applied over bare cold roll steel (CRS). Dry film thickness (DFT) of the clearcoat was approximately 1.0–1.2 mil. Tested film properties include MEK resistance,17 pencil hardness,18 pendulum hardness,19 crosshatch (CH) adhesion,20 and gloss.21
NACURE XC-324 provided similar cure response to the control catalysts using both bake schedules. Coatings catalyzed with 2-ethylimidazole exhibited the poorest gloss when baked at both temperatures while XC-324 catalyzed coatings provided high gloss properties, exhibiting similar gloss units (GU) to the octydimethylamine catalyzed system.
It should be acknowledged that a decrease in pencil hardness was observed for XC-324 when dropping the bake schedule to 110°C. However, XC-324 did not exhibit as significant of a loss in pendulum hardness or adhesion as the control films when baked at 110°C vs 120°C. Results are in Tables 3a and 3b.
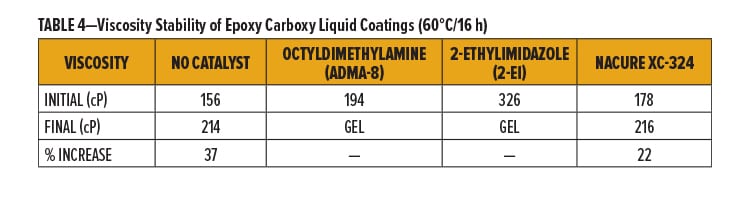
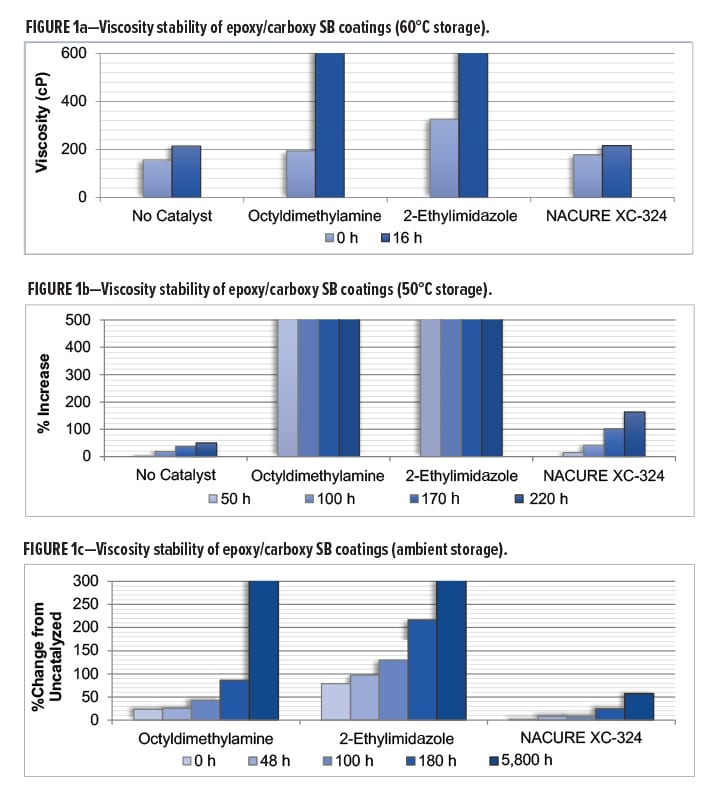
Viscosity Stability of Epoxy Carboxy Liquid Coatings
All 1K formulation should be able to remain stable during transportation and storage prior to end use application. To assess these properties, age stability of catalyzed epoxy/acid clearcoats was evaluated by monitoring viscosity change of the catalyzed systems upon aging using three storage conditions: 60°C, 50°C, and room temperature (RT).
NACURE XC-324 was evaluated against the octyldimethylamine and 2-ethylimidazole catalysts at all three storage temperatures. In addition, all three tests included an uncatalyzed system. Viscosities were measured using an AR1000 rheometer with a 40 mm 2° steel cone geometry from TA Instruments. Samples were tested at 25°C using a shear rate of 100 s-1.
NACURE XC-324 showed minimal change in viscosity during all age stability testing with significant improvement in viscosity stability compared to both control catalysts. Furthermore, the XC-324 catalyzed system behaved nearly the same as the uncatalyzed epoxy/carboxy system during most of the storage testing.
Results for viscosity stability using 60°C storage are in Table 4 and Figure 1a. Results for 50°C and RT (ambient) storage are in Figures 1b and 1c, respectively.
Gel Fraction Studies
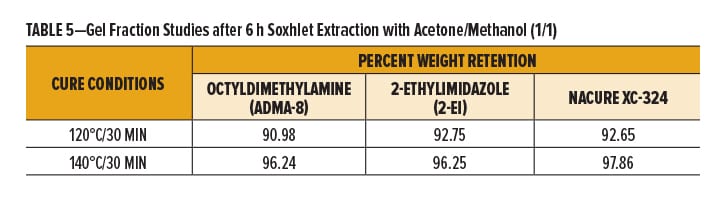
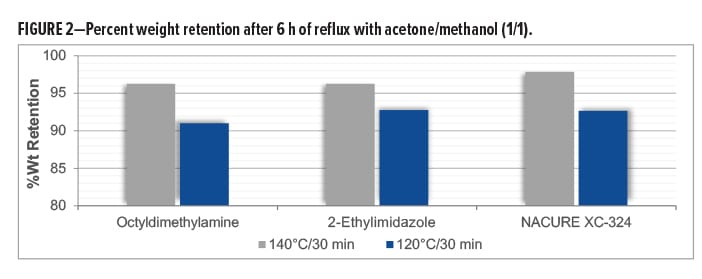
The cure response of a coating can be verified by measuring the gel fraction of the polymer system, namely the percentage of polymer chains that have reacted and are crosslinked to form the coating film. A higher gel fraction correlates to a better cure with more crosslinking. The gel fraction is defined as the weight ratio of dried network polymer (crosslinked material) to that of the polymer before being subjected to Soxhlet extraction conditions.
Gel fractions studies were conducted by subjecting 1K SB epoxy/carboxy clearcoats catalyzed with NACURE® XC-324, octyldimethylamine, and 2-ethylimidazole to Soxhlet extraction conditions. Formulations were catalyzed with 1.0% catalyst solids based on TRS. Epoxy/carboxy systems were prepared over an untreated thermoplastic polyolefin (TPO) substrate to facilitate easy removal of the cured film. Films were baked for 30 min at 140°C and 120°C. DFT of the clearcoats was approximately 1.0–1.2 mil.
A 3 in. x 6 in. specimen of cured film was placed inside a pre-weighed glass thimble. All thimbles were previously cleaned and dehydrated before weighing. The glassware was submerged in a 1:1 acetone/N,N-dimethylformamide (DMF) solution overnight and then dehydrated for one hour at 110°C. Initial tare weight of the thimble was determined after cooling at room temperature in a desiccator for approximately 20–30 min.
The thimble containing the sample was weighed and placed in the extraction chamber. A 1:1 acetone/methanol mixture was used as the extraction solvent. Films were subjected to a six-hour reflux. Films were then placed in the oven for one hour at 110°C to dehydrate and remove any residual solvent from the thimble and film. The thimble containing the dried film sample was reweighed after being cooled at room temperature in a desiccator for approximately 20–30 min.
Gel fractions were reported as percent weight retention, where weight retention is defined as the ratio of film weight after extraction to the initial weight before extraction.
NACURE XC-324 provided a high degree of crosslinking with the 140°C and 120°C bake, exhibiting a similar weight retention as both controls. Results are in Table 5 and Figure 2.
QUV Resistance of Catalyzed Epoxy Carboxy Coatings
Coating discoloration induced by catalysts upon UV exposure is an undesired property. Basic amine catalysts are known to contribute to yellowness as a result of such exposures.
A blocked sulfonic acid catalyzed melamine crosslinked WB white basecoat was applied over iron phosphated CRS and baked at 80°C/10 min. The catalyzed epoxy/carboxy clearcoats were then applied over the white basecoat and cured at 120°C/30 min. The DFT of the clearcoat was approximately 1.6–1.8 mil.
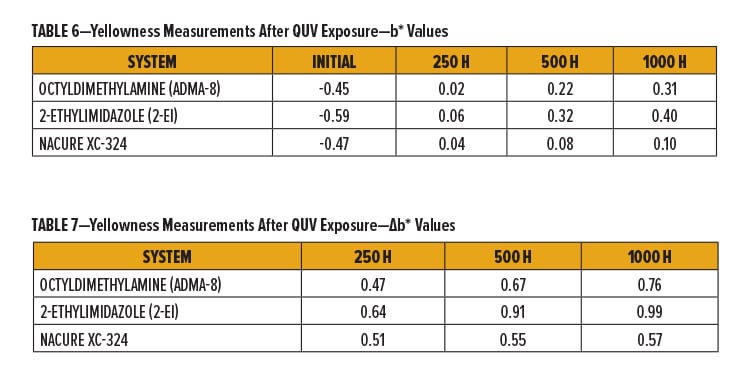
Cured films were evaluated for UV resistance by measurement of b* values of the panels after 250, 500, and 1000 h of QUV exposure.22
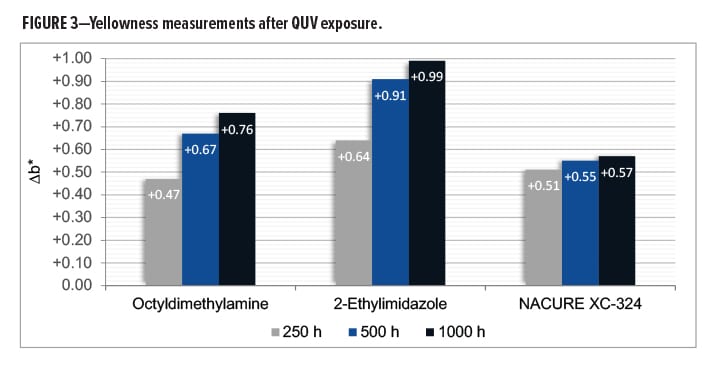
NACURE XC-324 provided a rather significant improvement in UV resistance compared to octyldimethylamine and 2-ethylimidazole, especially with regards to the longer QUV exposure times of 500 and 1000 h. Results are in Tables 6–7 and Figure 3.
Humidity Exposures of Catalyzed Epoxy Carboxy Coatings
Epoxy/carboxy clearcoats were applied over an electrocoated CRS substrate and baked at 120°C/30 min. DFT of the cured films was approximately 1.6–1.8 mil.
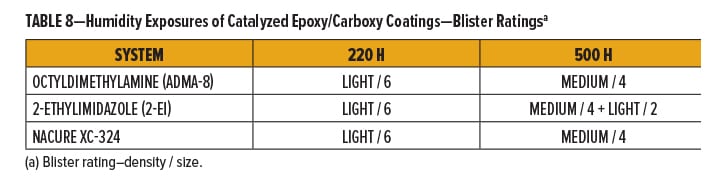
Cured films were exposed to Cleveland humidity conditions.23 The chamber was maintained at 100% RH through the experiment. Exposed films were evaluated for blistering. Films were given blister ratings24 following 220 and 500 h of humidity exposure.
NACURE XC-324 provided similar humidity resistance to octyldimethylamine. However, XC-324 showed an improvement compared to 2-ethylimidazole. Results are shown in Table 8.
Crockmeter Abrasion
Catalyzed clearcoats were applied over bare CRS and baked at 120°C/30 min. Baked films were tested for mar/scratch resistance using a Crockmeter abrasion tester (M238AA) from SDL Atlas. The DFT of the clearcoat was approximately 1.6–1.8 mil.
An abrasion dry test was performed on each of the coatings using 2 in. x 2 in. Crockmeter squares on the crock finger and 20 Mule Team Borax on the test specimen. Each of the coatings were subjected to 20 cycles of abrasion.
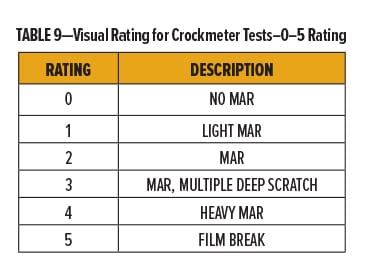
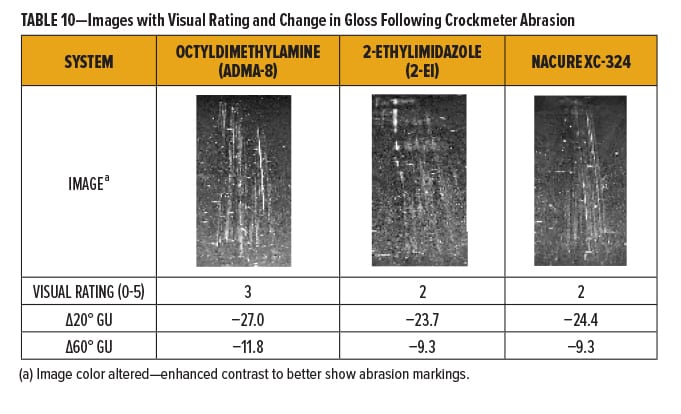
The degree of marring for each of the films was rated qualitatively using a 0–5 scale with “0” and “5” being “No Mar” and “Film Break,” respectively. Break down of the 0–5 scale is in Table 9. Films were also evaluated quantitatively by measuring the change in gloss following abrasion.
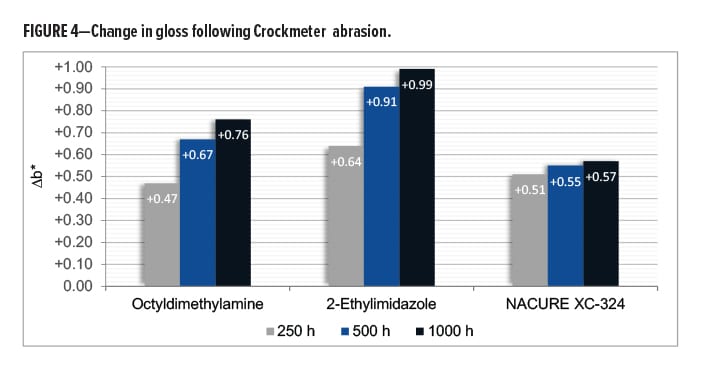
The NACURE XC-324 catalyzed film exhibited good resistance to Crockmeter abrasion in comparison to the control catalysts. XC-324 provided very similar abrasion resistance to 2-ethylimidazole. Nonetheless, the XC-324 films had a lower frequency of deep scratches and a lesser change in gloss than the films catalyzed by octyldimethylamine. Results are in Table 10 and Figure 4.
Overbake Resistance
A blocked sulfonic acid catalyzed melamine crosslinked WB white basecoat was applied over iron phosphated CRS and baked at 110°C/10 min. The catalyzed epoxy/carboxy clearcoats were then applied over the white basecoat and cured at 120°C/30 min. The DFT of the clearcoat was approximately 1.6–1.8 mil.
Catalyzed films were tested for overbake resistance by evaluating the coating’s color change. Films were subjected to three consecutive cycles of overbake: (1) 120°C/30 min, (2) 120°C/30 min and (3) 150°C/30 min.
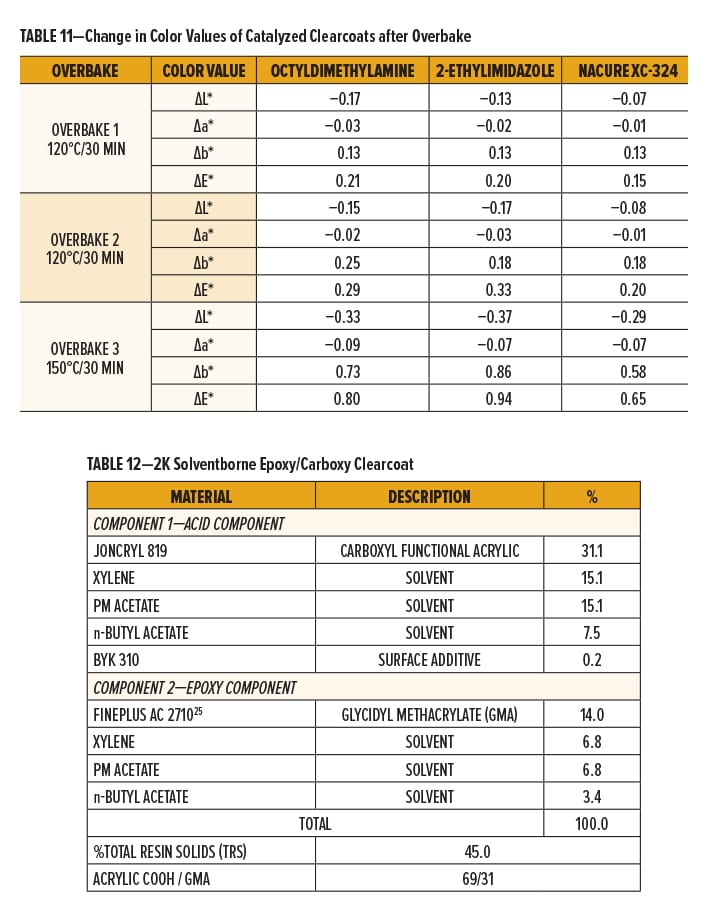
All clearcoats showed a minimal change in color following the first overbake cycle. However, relative to the control catalysts, NACURE XC-324 did exhibit a lesser change in total color (∆E*). A similar trend was observed for the second overbake cycle.
More significant color changes occurred following the third overbake cycle. The XC-324 films provided a significant improvement in resistance to yellowing (∆b) and exhibited a much lesser change in total color compared to the controls. Results are in Figure 5 and Table 11.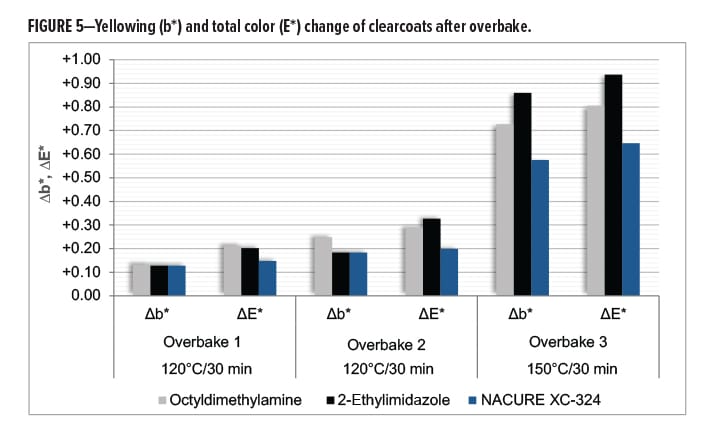
Experiment II: Catalyst Studies on NACURE XC-355 in a 2K SB Epoxy/Carboxy Clearcoat
NACURE XC-355 was evaluated in a 2K SB epoxy/carboxy clearcoat and tested for lower temperature cure capabilities.
Materials and Preparation of 2K SB Epoxy/Carboxy Clearcoat Formulation
The 2K SB epoxy/carboxy clearcoat formulation was prepared by dissolving Joncryl 819, a solid grade carboxyl functional acrylic resin, and Fineplus AC 2710,25 a solid grade glycidyl methacrylate resin, separately using a solvent blend containing xylene, PM acetate, and n-butyl acetate (40.1/40.1/19.8). The flow additive was stored in the acid component. Breakdown of formulation components is presented in Table 12.
The acid component, containing the carboxyl (COOH) functional acrylic, and the epoxy component, containing the glycidyl methacrylate (GMA) resin were both 45% TRS.
Catalysts were premixed into the acid component prior to addition of the epoxy component. All mixing was conducted via high speed dispersion using a FlackTek high speed mixer.
The clearcoat was formulated stoichiometrically. The two components were blended 69/31 by total formula weight to give a solids ratio of 69/31 Acrylic COOH/GMA. This solids ratio provides an approximately 1:1 molar ratio. The TRS of the final resin system was 45%.
Sample testing or film preparation began immediately after incorporating the epoxy component into the acid component.
Film Preparation of 2K SB Epoxy/Carboxy Clearcoat
All films prepared for testing were applied via draw down. Epoxy/carboxy clearcoats were applied using a drawn down bar. White WB basecoats used in color testing were applied using a bird applicator. Epoxy/carboxy clearcoats and WB basecoats were both given an approximately 10–15 min flash at ambient temperatures prior to baking at the designated temperatures.
Rheology Studies on Catalyst Activity at 100°C
An oscillation study on the change in complex viscosity (|h*|) was conducted using an AR2000 rheometer from TA Instruments. A 20 mm steel flat plate geometry was used during testing.
The uncatalyzed epoxy/carboxy clearcoat was compared to a system containing 1% NACURE XC-355 solids on TRS.
Oscillation testing was comprised of four steps following sample equilibration at 20°C: 1) Heat to 100°C, 2) Isotherm for 60 min, 3) Cool to 20°C, and 4) Re-heat to 100°C. The pre-experiment steps used for flashing solvent from the samples are shown in Table 13a. The protocol used for oscillation testing is in Table 13b.
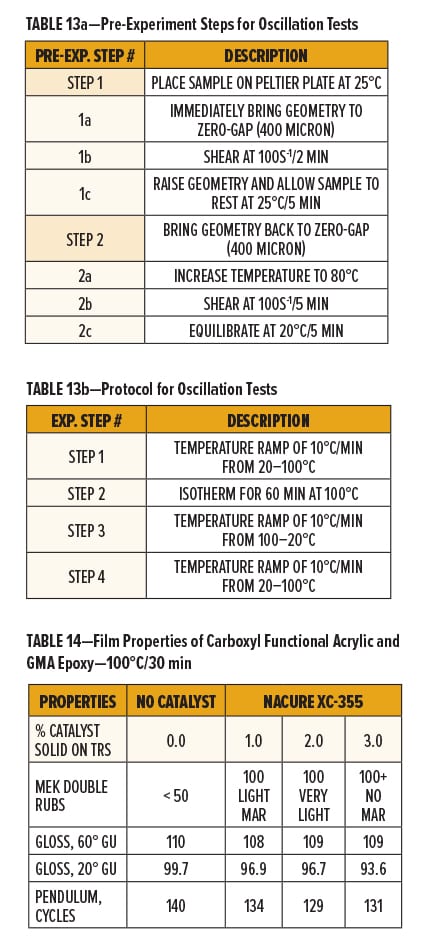
The uncatalyzed and NACURE XC-355 systems both showed a decrease in complex viscosity as the solid grade resins begin to soften or melt upon heating during the first temperature ramp to 100°C. It should be noted the rate of change in viscosity was slightly lower for the XC-355 system during the first temperature ramp. This could indicate that the catalyst had already activated the system at temperatures below 100°C.
Minimal increase in viscosity for the uncatalyzed epoxy/carboxy system occurred throughout the 60 min isotherm at 100°C. Catalyzing the formulation with NACURE XC-355 provided a significant increase in viscosity during the isotherm. It was also observed that the rate of change in viscosity of the XC-355 system was most significant during the first half of the isothermal step. The rate of change beginning to diminish or plateau could suggest that the epoxy/carboxyl crosslinking reaction was at least near to completion after approximately 30 min at 100°C.
The uncatalyzed system additionally exhibited a large increase followed by a large decrease in viscosity when cooling the sample to 20°C followed by re-heating to 100°C, respectively. This is caused by uncrosslinked resins re-solidifying to their solid state during cool down and again softening or re-melting upon additional heating.
On the contrary, the XC-355 system exhibited minimal change in viscosity during cool down or re-heating of the sample. The rheology of the resin system no longer exhibiting physical properties of the individual solid grade resins suggests that XC-355 successfully catalyzed the reaction, producing a more thermally stable crosslinked network following the isotherm at 100°C.
Data collected from these oscillation tests confirm that NACURE XC-355 is a high active catalyst for reaction of carboxyl and epoxy functional groups. Furthermore, the results validate XC-355 as a catalyst capable of promoting the epoxy/carboxyl reaction at bake temperatures of 100°C or potentially lower. Results are shown in Figure 6.
Film Properties of Catalyzed Clearcoats
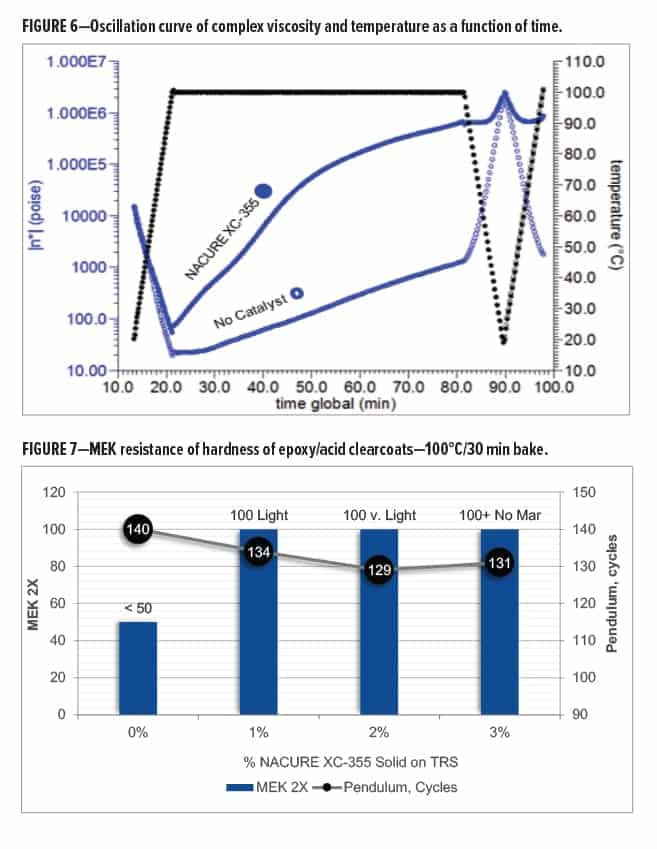
Epoxy/carboxy clearcoats catalyzed with increasing dosages of NACURE XC-355 were evaluated for MEK resistance, gloss, and pendulum hardness. Films were prepared over bare CRS and baked at 100°C/30 min. DFT of the clearcoats was approximately 1.0–1.2 mil.
Systems were catalyzed with 0, 1, 2, and 3% NACURE XC-355 solids on TRS.
All systems showed a very high pendulum hardness and great gloss. The NACURE XC-355 systems exhibited a slight decrease in both gloss and hardness. However, the system containing no catalyst gave very poor MEK resistance. This indicates that the uncatalyzed resin system was not fully crosslinked.
All NACURE XC-355 dosages provided great MEK resistance. The XC-355 systems all achieved 100 MEK 2X. Increasing catalyst dosage provided improved mar resistance. Results are in Table 14 and Figure 7.
Overbake Resistance of Catalyzed Clearcoats
A blocked sulfonic acid catalyzed melamine crosslinked white WB basecoat was applied over iron phosphated CRS and baked at 110°C/10 min. The catalyzed epoxy/carboxy clearcoats were then applied over the white basecoat and cured at 100°C/30 min. The DFT of the clearcoat was approximately 1.0–1.2 mil.
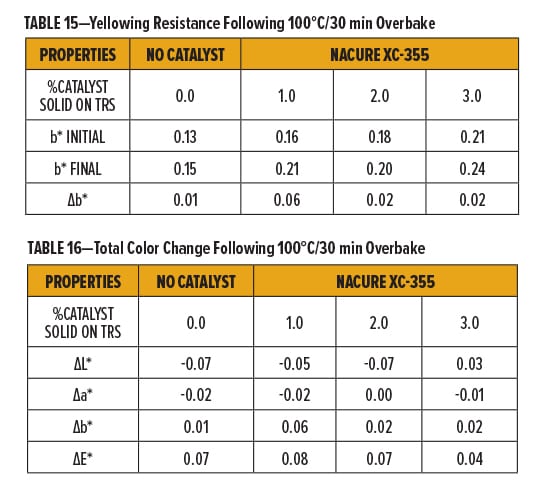
Films were overbaked at 100°C/30 min after testing initial color of the films. Color change was evaluated for clearcoats containing 0, 1, 2, and 3% NACURE XC-355 solids on TRS.
The clearcoats all exhibited very little yellowing (b*) following the initial 100°C/30min bake and the overbake. Although nearly negligible, very slight increases in yellowing after overbake for systems containing XC-355 were observed but it should be acknowledged that the uncatalyzed system was not fully cured as previously demonstrated. Additionally, minimal differences in total color (∆E*) following overbake occurred for all dosages of XC-355. Results are in Table 15 and Table 16.
Experiment III: Environmental Etch Resistance of Epoxy/Carboxy vs HMMM/OH
Environmental etch resistance of epoxy/carboxy clearcoats catalyzed with NACURE XC-355 was compared to an aminoplast crosslinked polyol clearcoat catalyzed with amine blocked p-TSA.
Materials and Preparation of SB Epoxy/Carboxy and HMMM/OH Clearcoat Formulations
The two formulations (A and B) were both formulated as 2K systems. Formulation A uses a solid grade hydroxyl (OH) functional acrylic resin (Component 1) for crosslinking with hexa(methoxymethyl)melamine (HMMM) (Component 2). Formulation B uses a solid grade carboxyl (COOH) functional acrylic resin (Component 1) for crosslinking with a GMA resin (Component 2).
Components 1 and 2 of Formulation A and B were formulated to 45% TRS by dissolving the resins in a solvent blend containing xylene, PM acetate, and n-butyl acetate (40.1/40.1/19.8). Flow additive was stored in Component I of both formulations. Breakdown of formulation components is in Table 17.
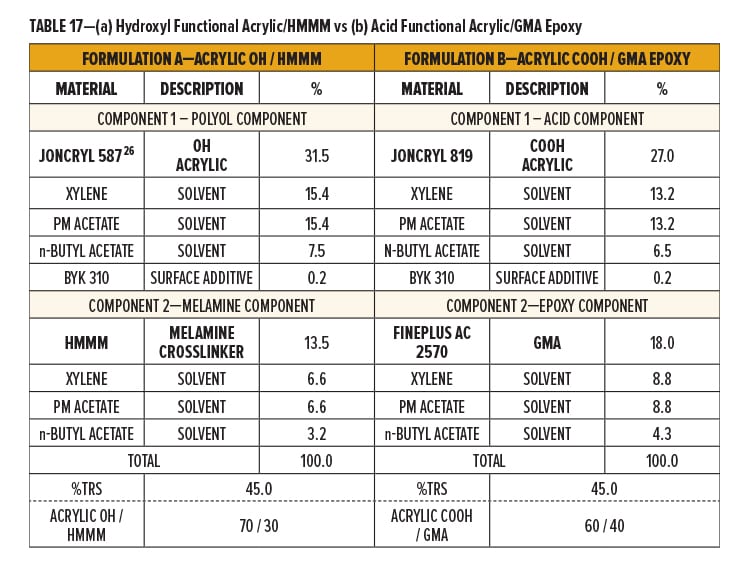
Formulation A is based on an experimentally optimized ratio of solid resin. The two components were blended 70/30 to give a solid ratio of 70/30 Acrylic OH/HMMM. The TRS of the final resin system was 45%.
Formulation B was formulated stoichiometrically. The two components were blended 60/40 by total formula weight to give a solids ratio of 60/40 Acrylic COOH/GMA. This solids ratio provides an approximately 1:1 molar ratio. The TRS of the final resin system was 45%.
Catalyst was premixed into Component I of both Formulation A and B. Formulation A, the Acrylic OH/HMMM system, was catalyzed with an amine neutralized p-TSA (0.56% p-TSA on TRS or 1% as supplied on TFW). Formulation B, the Acrylic COOH/GMA system, was catalyzed with NACURE XC-355 (1.0% solids on TRS or 0.9% as supplied on TFW).
For both Formulation A and B, Component II was incorporated into Component I containing the pre-catalyzed acrylic resin solutions. Catalyst premixing and component incorporation was conducted via high speed dispersion using a FlackTek high speed mixer.
Sample testing or film preparation began immediately after incorporating the HMMM and GMA solutions to the OH Acrylic and COOH Acrylic solution, respectively.
Film Preparation of HMMM Crosslinked Acrylic and Epoxy/Carboxy Clearcoats
Films were applied over bare CRS via draw down, given an approximately 10–15 min flash at room temperature, and baked at 120°C/30 min. Epoxy/carboxy and aminoplast crosslinked films were tested for cure response and environmental etch resistance once cooled to room temperature following bake.
Cure Response of Acrylic OH/HMMM and Acrylic COOH/GMA
Cure responses of the Acrylic OH/HMMM (Formulation A) and the Acrylic COOH/GMA (Formulation B) were tested by evaluating the film’s resistance to MEK.
Formulation A and B both exhibited great cure response using the designated catalyst dosages and bake schedule. Both formulations achieved 100 MEK 2X with minimal marring. Results are shown in Table 18.
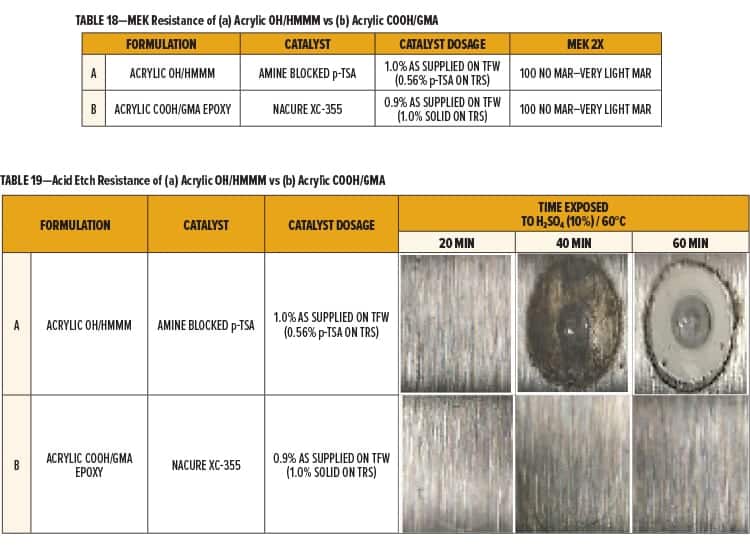
Environmental Etch Comparison of Acrylic OH/HMMM vs Acrylic COOH/GMA
Environmental etch of the Acrylic OH/HMMM (Formulation A) and the Acrylic COOH/GMA (Formulation B) was evaluated by subjecting the films to a diluted solution of sulfuric acid (H2SO4) at elevated temperatures.
Equal amounts of a 10% H2SO4 solution were placed onto the film in three spots of equal surface area. Test areas were covered, placed in an oven at 60°C, and evaluated in 20-min intervals. The acid solution was rinsed off the panel using dIH2O once the films were near room temperature. Excess acid or dIH2O was removed by lightly pressing with a towel.
The Acrylic OH/HMMM system catalyzed with amine neutralized p-TSA (Formulation A) began to fail following 40 min of testing. Precipitate and a blister in the center of the test area was observed for the HMMM crosslinked system after 60 min of testing.
The Acrylic COOH/GMA system catalyzed with NACURE XC-355 exhibited superior acid etch resistance, showing only slight markings following 60 min of acid exposure. Results are given in Table 19.
SUMMARY AND CONCLUSIONS
The new class of metal-free catalysts are found to be very effective in promoting the crosslinking reaction of carboxyl functional polymers with epoxy functional resins with potential utility in automotive clearcoats and powder coating applications.
NACURE XC-324 was determined to be a versatile stable catalyst that can effectively promote the crosslinking reaction of a 1K SB epoxy/carboxy clearcoat using bake temperatures as low as 110–120°C. In addition to good cure with exceptional age stability, XC-324 films exhibited high gloss, great hardness, low color, and good adhesion to metal, as well as excellent overbake, humidity, UV, and abrasion resistance.
Investigations on lower temperature cure response validated NACURE XC-355 as a high active catalyst capable of providing good cure of 2K SB epoxy/carboxy clearcoats using bake temperatures of 100°C or potentially lower. In addition to excellent cure response, XC-355 films exhibited high gloss and great hardness as well as low color with good overbake resistance even at higher catalyst dosages.
Environmental etch studies conducted on hydroxyl vs carboxyl functional acrylic resins crosslinked with HMMM vs a GMA and catalyzed with amine neutralized p-TSA vs XC-355, respectively, demonstrated that epoxy/carboxy clearcoats can likely be used as an alternative to aminoplast crosslinked clearcoats when applications require superior acid etch resistance.
ACKNOWLEDGMENTS
We thank Morgan Alexander for experimental support and for evaluations of the new catalysts in various coating systems. We thank King Industries for permission to publish this work.
References
1. Schulz, U., Trubiroha, P., Schernau, U., and Baumgart, H., “The Effects of Acid Rain on the Appearance of Automotive Paint Systems Studied Outdoors and in a New Artificial Weathering Test,” Prog. Org. Coat., 40, 151-165 (2000).
2. Palm, M. and Carlsson, B., “New Accelerated Weathering Tests Including Acid Rains,” J. Coat. Technol., 74, 69-74 (2002).
3. Trinidade, D.J., Matheson, R.R., U.S. Patent 8710138; Barsotti, R.J., Hazan, I., and Nordstrom, J.D., U.S. Patent 6428898 (2002); Groenewolt, M., Hesener, S., Stubbe, W., Niemeier, M., and Westhoff, L., U.S. Patent 8658752 (2014).
4. Edwards, P.A., Striemer, G., and Webster, D.C., “Novel polyurethane coating technology through glycidyl carbamate chemistry,” J. Coat. Technol. Res., 2 (7), 517-527 (2005).
5. Wulfsohn, W. and Knavish, T., “Automotive OEM Coatings,” PPG Industries Presentation, Feb. 2010.
6. Merefeld, G., Molaison, C., Koeniger, R., Ascar, A.E., Mordhorst, S., Suriano, J., Warner, R.S., Gray, K., Kovaleski, K., Garrett, G., Finley, S., Meredith, D., Spicer, M., and Naguy, T., “Acid/Epoxy Reaction Catalyst Screening for Low Temperature (120°C) Powder Coatings,” Prog. Org. Coat., 52, 98-109 (2005).
7. Global Powder Coatings Market Report, Acmite Market Intelligence, September 2011.
8. Brun, L. and Golini, R., “Powder Coating: A Global Value Chain Perspective,” Powder Coating, 21, 29-33 (2010).
9. Flosbach, C. and Schubert, W., “Zero Etch Clear—A New Modular Clearcoat System with Excellent Scratch/Mar Performance,” Prog. Org. Coat., 43, 123-130 (2001).
10. Igrashi, H., Kitagawa, H., Okumura, Y., Saika, M., Nagai, K., and Yabuta, M., Kansai Paint Co., Ltd., U.S. Patent 6,214,418 (2001).
11. Blank, W.J., He, Z.A., and Picci, M., “Catalysis of the Epoxy-Carboxyl Reaction,” J. Coat. Technol., 74, 33-41 (2002).
12. Blank, W.J., “Advances in Catalysis for Organic Coatings,” Chimia, 56, 191-196 (2002).
13. Subrayan, R.P., Miller D.J., Emmet M.M., and Blank, W.J., “Catalysis of Thermally Curable High Solids Cycloaliphatic Epoxy Formulations,” ACS PMSE Preprints, Chicago Meeting, 2001.
14. Joncryl 819 solid grade acrylic polymer from BASF with a carboxyl equivalent weight of 748.
15. Fineplus AC 2570 solid grade glycidyl methacrylate from DIC Performance Resins, with an epoxy equivalent weight of 480 based on resin solids.
16. BYK-310 Silicone surface additive from BYK.
17. ASTM D4752 modified, Rub with MEK Soaked Cloth Attached to Pound Ball Peen Hammer.
18. ASTM D3363, Test Method for Film Hardness by Pencil Test.
19. ASTM 4366, Standard Test Methods for Hardness of Organic Coatings by Pendulum Damping Tests.
20. ASTM D3359, Standard Test Methods for Measuring Adhesion by Tape Test.
21. ASTM D2457, Standard Test Method for Specular Gloss of Plastic Films and Solid Plastics.
22. ASTM D4587-11, Standard Practice for Fluorescent UV-Condensation Exposures of Paint and Related Coatings.
23. ASTM D2247, Standard Practice for Testing Water Resistance of Coatings in 100% Relative Humidity.
24. ASTM D714, Standard Test Method for Evaluating Degree of Blistering of Paints.
25. Fineplus AC 2710 solid grade glycidyl methacrylate from DIC Performance Resins, with an epoxy equivalent weight of 343 based on resin solids.
26. Joncryl 587 solid grade acrylic polymer from BASF with a hydroxyl equivalent weight of 610.
Presented at the American Coatings Conference, April 9-11, 2018 in Indianapolis, IN.
CoatingsTech | Vol. 16, No. 3 | March, 2018
