By M. Gessner, A. Nanda, R. Subramanian, D. Sauer, W. DeGooyer, E. McCracken, and L. Lindsey, Allnex USA and F.G.H. van Wijk, Allnex, Netherlands
In 2015, the breakthrough of a novel blocked catalyst and kinetic control additive package used in conjunction with Michael Addition chemistry was announced at the European Coating Conference.1 Prototype paint formulae highlight the very fast drying 2K system (<15 min, ambient) and the unique de-coupling of dry time and pot life (>5 h), expected to trigger new paint and process solutions. The fast-cure system was designed as a topcoat over epoxy primers for use in the industrial market. The advantages of fast cure, long pot life, and low- to no-baking were described at other conferences since the introduction. However, most factory-applied systems do not fully make use of these advantages.
Air-dry-only applications fully use the advantages of this new technology. Furthermore, the base-catalyzed system is a natural consideration for use over alkaline surfaces such as concrete. Very low-VOC/air-dried coatings can be made using reactive diluents, which can reduce paint viscosity, but also greatly increase the crosslink density. There are also available low-viscosity crosslinkers, which reduce the viscosity of the paint, yield good paint properties, and have excellent appearance. This article shows the low-VOC potential while the fast-dry nature allows fast return-to-service for flooring and other applications.
INTRODUCTION
Driven by changes in Health/Safety/Environmental (HSE) legislation as well as competition on paint application costs and coating performance, today’s paint technology continues to develop with focus on isocyanate-free, higher solids, lower curing temperatures and faster painting processes. Meeting these combined requirements is challenging and encroaches on the versatility limits of presently available cure chemistries. Michael Addition chemistry2 opens a prospective route for making steps beyond these limits.
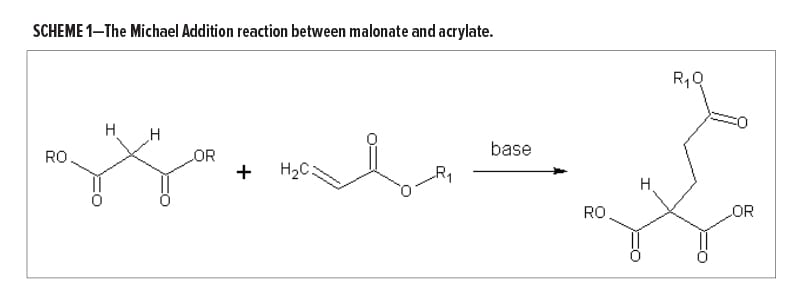
Michael Addition (MA) has been previously explored and used in coatings applications3; however, it has never established itself as a mainstream technology, primarily because it is too reactive. The key components of an MA system are electron deficient C=C double bonds (e.g., an acryloyl, the acceptor), acidic C–H bonds (as present in acetoacetate and malonate moieties, the donor), and a base catalyst strong enough to abstract the proton of this C–H bond yielding a nucleophilic carbanion that can add to the double bond. A carbon–carbon link is formed between the two molecules. The second proton of the donor species is available for reaction with similar reactivity (Scheme 1).
Relevant features derived from the characteristics of MA chemistry include:
- The need for a base, strong enough to abstract a proton from the donor species. The pKa of an acetoacetate C–H is around 10.7; for a malonate, it is even higher (>13).
- The absence of acidic species, which would deactivate the catalyst.
- The very high reactivity of the carbanions formed, especially when using malonate species as the donor. In paint formulations, it is easy to create conditions under which an MA reaction between malonate and acryloyl species can essentially be completed within minutes. In this instance, both malonate and acryloyl may coexist in the uncatalyzed paint and present good shelf stability.
- The nature of the carbon–carbon links formed; not leading to weak spots in durability.
- MA technology opens a window to using nonpolar, low equivalent weight crosslinking components that can lead to very low solvent demand formulations capable of creating high crosslink density polymer networks.
The options of MA chemistry have been expanded by focusing on ways to control the inherent reactivity of a malonate–acryloyl system, using its high reactivity potential while creating both a long pot life (time for the viscosity to double) and a workable open time. Combined with specially developed resins, the obtained benefits are so profound that it may be recognized as a new type of curing technology, with potential for use in many different markets and applications.3 In this article, the new technology will be referred to as FCT (Fast Cure Technology). This technology is commercially available under the tradename Acure™.
Controlling Pot Life and Drying
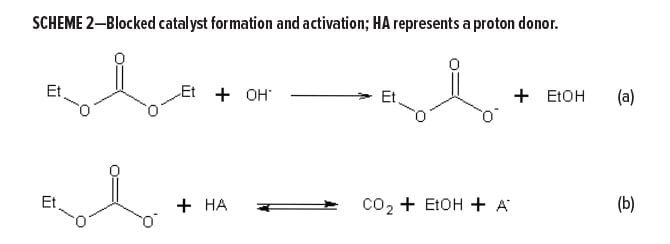
Very high reactivity cannot easily be combined with a good pot life. (Pot life refers to the time required for the viscosity to double.) However, a solution was found in the reversible blocking of a strong base catalyst with dialkyl–carbonate, as depicted in Scheme 2a. Strong bases will form alkyl carbonate anions with a basicity low enough to not initiate the MA reaction. These carbonates are inherently unstable, and through protonated species, will form an equilibrium with free CO2 and alcohol (Scheme 2b).
After mixing in a container (with a relatively low surface area/volume ratio) CO2 and alcohol release occurs extremely slowly and long pot lives are observed. Upon applying the paint, however, large surface areas will be created, facilitating the easy escape of solvent and, importantly, CO2. This shifts the equilibria, and is followed by rapid de-blocking of the basic species that triggers the full reactivity potential of the malonate acryloyl system. This de-blocking process will be accelerated when HA becomes more acidic, shifting equilibrium (Scheme 2b) to the right. The net result is the combination of a very long pot life with a very fast dry-through time. Workable pot lives of at least 4 h are easily obtained, and if needed, can be formulated to be counted in days. At the same time, we observe tack-free times down to 10 min after application, and stage-4 (dry through) drying recorder times not much longer. Figure 1 shows the different pot life/drying speed balance for FCT vs isocyanate-based systems.
The effect of adding volatile alcohols to FCT-based paints is given in Figure 2, showing pot life prolongation without significantly affecting dry times.
Tuning the reaction time is possible with the use of kinetic additives besides alcohol. Figure 3 shows the pKa of several materials that can be used in Michael Addition. Selecting a material with a pKa lower than malonate will make that material the first to be de-protonated. FCT paints typically use succinimide and 1,2,4-triazole as part of the formulation for the sake of balancing cure speed, open time, and pot life. The low pKa of benzo-triazole explains why UV absorbers based on substituted benzo-triazoles are avoided.
FCT paints consist of two components. Component A contains malonated resin, acryloyl crosslinker(s), kinetic additives including alcohol, dispersed pigment (in the case of pigmented paints), solvent(s), and typical paint additives (light stabilizers, surface active agents, etc.), which are stable until the addition of the catalyst, which is Component B.
The conversion of acryloyl post-catalyzation can be followed by observing the absorption of the double bond using FTIR (809 cm-1). Figure 4 shows the FTIR spectra of three ratios of acceptor (acryloyl) to C–H before and after catalyzation. The percent residual acryloyl is calculated from the area per equation (1).
– % Residual acryloyl double bonds= (A t /A 0)*100 % (1)
eq (1): Determination of residual double bonds at time=0 (A0) and t (At)
Formulating for Low-VOC Paints
Malonated resins are made at very low acid value and have low hydroxyl content, which leads them to be low in polarity and thus lower in viscosity than resins normally used in 2K paints. Even with low polarity, molecular weight plays a role in formulating low viscosity and thus low-VOC paints, which will be shown. However, it is also useful to develop tools besides molecular weight to reduce viscosity, raise application solids, and lower VOC.
FCT resins can be functionalized with either dimethyl malonate (DMM) or diethyl malonate (DEM). In the case of air-dried paints, the low volatility of DMM and DEM can allow them to be used as reactive diluents (RD). The extent to which they will react with acryloyl functionality and not be fugitive and thus become part of the solids needs to be determined.
Reactive diluents are well known in the paint industry and have been used for many years. They tend to be molecules with relatively low molecular weights or oligomers, sometimes of the resins themselves. The same concept can be extended to FCT paints; malonate functional oligomeric RDs can be made from simple di- and triols to yield low viscosity materials, but crosslinkable into the paint matrix. Molecules can be selected that will add hardness, flexibility, or increase crosslink density as well.
EXPERIMENTAL
Diethyl Malonate
Leaving residual DEM in the resin or using it solely as a reactable moity is a good idea if it can be shown to thoroughly react and not volatilize and thus be counted as a VOC. The solids content of DEM, trimethylolpropane triacrylate (TMPTA), and di-trimethylolpropane tetraacrylate (DiTMPTA) were determined per ASTM D-2369 to 0, 88.0, and 99.78%, respectively, using a 110˚C oven.
Samples were formulated with DEM and TMPTA or DiTMPTA at double-bond to C–H ratios (A/D ratio or stoichiometry) from 0.5:1 to 1:1. The samples were catalyzed with an unblocked version of the FCT catalyst and weighed into aluminum pans. The samples were allowed to cure for 18 h and then half were put into an oven at 110˚C for an hour. The baked pans and unbaked pans were then weighed and the solids content determined. The unbaked pans and the uncatalzyed samples were tested for residual double bonds per the FTIR method above. Figure 5 shows the data for the DEM/TMPTA and DEM/DiTMPTA samples. The theoretical solids were calculated assuming that all of the DEM would react with the acryloyl, thus becoming nonvolatile; the volatiles would be the solvent coming into the samples from the catalyst itself.
When the stoichiometry is 0.5:1, some of the DEM will have one hydrogen removed and replaced by a bond to the acryloyl. Some of the DEM can have both hydrogens removed and replaced by bonds to the acryloyl and other DEM molecules will be completely unreacted, thus leading to the baked solids being lower than the theoretical solids. When the stoichiometry is 1:1, each C–H has a potential to react. Thus, the baked solids should be within experimental error of the theoretical solids, which it is. Above a stoichiometry of 0.8:1, each DEM is at least reacted with one acryloyl. This is the case with either TMPTA or DiTMPTA. The FTIR analysis shows the maximum amount of unreacted double bonds to be 1%, which is within the error of the technique. The concept of using DEM or DMM as a reactive solvent is valid for air-dried paints.
Malonate Functional Reactive Diluents
Reactive diluents were made by reacting DEM with methyl propane diol (MPD), cyclohexyldimethanol (CHDM), hexane diol (HDO), ethylene glycol (EG), and a blend of MPD and trimethylolpropane (TMP) at a ratio of 1:1.5. Ethanol was removed during the synthesis and the residual DEM and viscosity measured. Table 1 shows the oligomers made and their theoretical C–H equivalent weights. An acetoacetate functional oligomer, 510-400, was also included in this study. The CHDM made a high viscosity oligomer. The sample containing TMP was the next highest in viscosity due to some branching.
The RDs were formulated into paints with DiTMPTA at a stoichiometry of 1:1. The paints were catalyzed and air-dried, which would consume any residual DEM via reaction with the acryloyl. All the paints made tack-free films. Figure 6 shows the viscosity of the paints and the hardness of the cured films as measured by Koenig pendulum hardness (KPH–sec). The viscosity of the paints followed the viscosity of the reactive diluents with CHDM making the most viscous paint. CHDM also made the hardest films and HDO and EG made the softest films.
Next, a malonated polyester resin, used in FCT paints, was used to make paints with the diluents in which 30% of the C–H functional sites came from the RDs. DiTMPTA was used as the acceptor at a stoichiometry of 1:1 donor to acceptor ratio. Figure 7 shows that all the RDs reduce the viscosity of the paint when compared to the control. All the oligomers increase the hardness except for the one based on HDO, which is the softest film. The acetoacetate functional oligomer, 510–400, makes the hardest film; it also has the lowest equivalent weight and the highest functionality.
100% Reactive and Lower Molecular Weight Resin
The next step toward low VOC is to make 100% reactive and lower molecular weight malonated resins. The standard FCT malonated polyester was made 100% reactive by eliminating the solids reduction step at the end of the synthesis; the viscosity is >600,000 cP. A lower molecular weight version was made. Figure 8 shows the GPC traces for the two resins. These two resins were used with the acetoacetate functional oligomer and the EG–DEM based oligomer to formulate paints using DiTMPTA as the acceptor at a 1:1 stoichiometry and at 92.5% paint solids. Table 2 shows the molecular weights (Mw), functionalities (Fn), and viscosities for the two resins and the selected RDs.
The effect that lowering the molecular weight of the resin has on paint viscosity is as expected. Figure 9 shows the viscosity and hardness of the paint. The dry-through time is a little bit longer, but not as much as could be expected (since the Mn has dropped by 500 Daltons), and it still dries in less than a half hour. The hardness is nearly the same, but the MEK resistance has decreased quite a bit (see Figure 10).
Improving Performance with Reactive Diluents
Two of the aforementioned RDs were selected for use with the 100% reactive standard molecular weight resin and the low molecular weight version. The acetoacetate functional resin, 510–400, has the highest functionality and the lowest equivalent weight that should increase crosslink density, increase hardness, and reduce paint viscosity. The EG–DEM based oligomer, 858–79, has the lowest viscosity, the second lowest equivalent weight, and has shown it can maintain hardness with the standard resin. All the paints were formulated at a stoichiometry of 1:1 with 1 mole of succinimide and 2 moles of 1,2,4-triazole per mole of catalyst, using TMPTA as the acryloyl, at a part A solids of 92.5% with 30% of the C–H functionality coming from the reactive diluent.
The standard resin, being the highest in viscosity, needs the reactive diluents the most. Figure 11a shows the viscosity dropping to less than half with either RD; the hardness increases when it is used. In Figure 11c, it is clear that the hardness increases as the % conversion of the double bonds increases. The RDs reduce the paint viscosity, but they are more accessible for reaction; thus, the stage 1 and 2 dry times are essentially the same and only the stage 3 and 4 dry times are longer. The MEK resistance was measured, but all of them were over 500 double rubs.
The reduced molecular weight resin is much lower in viscosity than the standard resin, but it too benefits from the low viscosity of the RDs. Figure 12a shows the viscosity dropping to less than half with either RD. The dry time stages remain nearly the same for all the samples (Figure 12B). In Figure 12c, it is shown that the hardness increases when the higher functionality and low equivalent weight RD is used, but is not changed by the EG–DEM based RD; the former contributes more crosslink density than the latter. The result of the increased % conversion with either of the RDs is reflected in the increase in MEK resistance (Figure 12d).
Formulating Low-VOC Floor Coatings
The path to high solids, low-VOC, and low-viscosity floor coatings can now be put together. A resin designed with high flow was made at 92% solids with DMM as a reactive solvent. The high functionality/low equivalent weight RD is used as part of the donor. Two gray paints were made at 0.8 P/B, one with the standard resin without any of the tools discussed and the second using the tools with the high-flow resin. Table 3 shows the paints applied to an old concrete floor in an area that experiences continuous fork-lift traffic.
The application viscosity of the standard paint is lower than high-flow paint, but raising it to the same viscosity would not bring the solids from 78% to the 94.5% of the high-flow paint or the 100 g/l VOC. Hardness is a little lower for the high-flow paint, but it has the same resistance to gouging/scraping, measured by the “five finger” tester, as the standard paint, 5N. The real test is how it holds up to constant traffic and wear.
Figure 13 shows the test area coated. The warehouse is over 20 years old and the concrete floors had never been painted. The floors were ground with a diamond-tipped pad to remove the years of dirt and grime. The cleaned concrete was divided into three sections with two different primers applied, separated by an area that was not primed. The next day, the two FCT topcoats were applied in strips separated by a commercially available polyurethane concrete topcoat. The low-VOC FCT topcoat (high-flow) was applied on the right with the higher VOC topcoat on the left. The picture on the left is before washing; marks from the fork-lift tires can be seen. A simple string mop and a soap solution was used to clean the floors, and this is shown on the right. The high-flow formula had the highest gloss, which can be seen in the reflection of the lights at the top of the picture. The high-flow paint also cleans easier than the standard FCT paint or the polyurethane control.
Figure 14 is a view along the traffic flow. The better removal of dirt from the high-flow paint can easily be seen, which is due to the composition of the main donor resin.
Figure 15 shows the same floors, but from the opposite direction. On the right is a close-up showing a pattern of dirt that ends sharply in the middle of the image. The paint layers in the bottom half are a primer and the FCT topcoat. The top half of the image has only FCT topcoat directly to concrete; a thickness of about 3–4 mils. The primer (a thickness of about 3–4 mils) serves to fill in the profile of the abraded concrete resulting in a smoother topcoat. The top half still has a rough profile in which the dirt remains since the string mop could not remove it due to the profile.
SUMMARIZING FCT PERFORMANCE
In this article, we have reviewed the chemistry of FCT paints and the use of reactive monomers, diluents, and molecular weight control to reduce VOC. This technology, based on Michael Addition chemistry, offers an impressive set of attractive performance parameters. Key features include: very fast drying, with fast crosslink density development, and very long pot life; application at ambient temperatures, or even below; very low solvent content (VOC < 250 g/L); and thick layer application (> 150 mm) possible. In addition, the technology offers very good chemical resistance; very good mar resistance; and it is an isocyanate-, formaldehyde-, and organotin-free cure chemistry.
We have reported on the validity of using excess diethyl malonate or dimethyl malonate as reactive monomers for malonated resins. Reactive diluents that reduce paint viscosity and improve % conversion and paint properties were discussed. The effect of molecular weight on paint viscosity and thereby VOC was shown and the use of reactive diluents with low molecular weight resins to restore chemical resistance. Lastly, we have formulated low-VOC floor paints that are in constant use and have shown excellent performance.
ACKNOWLEDGMENTS
The authors would like to thank Allnex management for allowing us to publish this paper. We would also like to acknowledge the contributions of colleagues at Allnex’s European laboratories in Bergen op Zoom, the Netherlands.
References
- van Wijk, F., et al., “Taming the Michael Addition Reaction,” Proc. European Coatings Conf., April 2015.
- Michael, A., “Ueber die Addition von Natriumacetessig- und Natriummalonsäureäthern zu den Aethern ungesättigter Säuren.” J. Praktische Chemie, 35, 349–356 (1887).
- Noomen, A., Prog. Org. Coat., 32, 137-142 (1997).
Presented at the 2017 CoatingsTech Conference, sponsored by ACA, March 20-22, in Cleveland, OH.
CoatingsTech | Vol. 14, No. 8 | August 2017