By Jacob D. Shevrin and Sheba D. Bergman, Evonik Corporation
As global environmental concerns continue to overshadow the use of well-established metal surface pretreatment processes such as chromate treatment and phosphatization, the need for environmentally friendly corrosion protection systems has never been greater. A promising solution to this worldwide regulatory issue is waterborne silane technology, which can offer a heavy metal-free, volatile organic compound (VOC)-free alternative to protecting metals from corrosion. The mechanism behind this corrosion protection can best be explained by the passivation of a metal surface with a waterborne silane film, which acts as a barrier to water, salts, and other corroding materials in the surrounding environment. It is important to note that the waterborne silane technology investigated in this work can be viewed as a type of conversion coating or pretreatment to the metal surface, rather than a conventional waterborne coating or primer. Certain waterborne silane technology requires high-temperature curing procedures for optimal results, which can be difficult to achieve in certain applications or industries. With the use of bipodal silanes, the additional crosslinking introduced into the system can alleviate the need for this high-temperature curing procedure. In this novel work, we demonstrate that the incorporation of a bipodal silane into waterborne silane systems improves the surface passivation of the metal surface, enhances the hydrophobicity of the system, and increases the crosslinking density of the system, leading to significant improvements in the corrosion resistance of waterborne silane technology.
INTRODUCTION
Whether it be for a bridge, a tunnel, an automobile, an electronic component, or a building, corrosion protection technology plays one of the most important roles in maintaining the integrity and longevity of the world around us. There are many well-established methods for protecting metals from corroding over time, including chromate treatment and phosphatization, which have been widely used for corrosion protection across the globe for decades.1,2 While these processes are inexpensive and well-known, governmental regulations and overall awareness of the hazards associated with these methods are growing. In particular, hexavalent chromium, a key material used in chromate treatment for the past 90 years, has recently been subject to new far-reaching restrictions. After the European Union classified hexavalent chromium as a carcinogen and mutagen in 2013, Europe’s Registration, Evaluation, Authorisation & Restriction of Chemicals (REACH) regulations have forced hexavalent chromium to be phased out of most industry applications across Europe. While most industries had to stop using hexavalent chromium by January 2019, some industries, such as the aerospace industry, have been allowed to continue using hexavalent chromium through 2026. However, corrosion protection technology for aerospace applications takes several years of research, development, and qualification, which is why the time for investigating chromium-free corrosion protection technology is now.
One viable alternative to these hazardous corrosion protection systems is silane technology. While organofunctional silanes have been widely used as adhesion promoters for several decades now, the use of these materials in corrosion resistant coatings is a more recent development. When properly prepared and applied, organofunctional silane coatings have the ability to form protective barriers on metal substrates, which subsequently protect the metals from corroding over time.3 Previous studies have shown that a only a small amount of active silane content, ranging from 0.2–2.0 wt% solids, is necessary for improving the adhesion of a coating system.4 For this reason, the incorporation of an organofunctional silane into a coating system can provide adhesion promotion or corrosion resistance without significantly increasing the volatile organic content (VOC) of the system. This is one of the many reasons why waterborne silane technology offers excellent corrosion resistance performance without the need for hazardous pretreatments, volatile solvents, or heavy metals.
The mechanism behind an organofunctional silane adhering to a metal surface is an important process to understand before investigating the corrosion resistance performance of waterborne silane technology. Over the past several decades, organofunctional silanes have been used as coupling agents for organic and inorganic materials across many different industries. Organofunctional silanes contain a hydrolyzable alkoxysilane (Si–OR) functional group that can bond with inorganic surfaces. In this work, the organofunctional silanes to be investigated have silicon functional groups comprising of alkoxy groups, specifically methoxy and ethoxy groups. Organofunctional silanes also consist of an organofunctional group that can react with organic systems. The simultaneous reaction of the silicon functional groups and organofunctional groups allow organofunctional silanes to act as an adhesion promoter between inorganic and organic materials.
For an organofunctional silane-based system to adhere to an inorganic substrate, hydrolysis must first take place at the alkoxy sites to form silanol groups. When the hydrolyzed organofunctional silane comes into contact with an inorganic surface, the silanol groups can initially form hydrogen bonds with the hydroxyl groups on the inorganic surface. Upon removal of moisture from the system, these hydrogen bonds can form siloxane bonds between the organofunctional silane and inorganic surface. These siloxane bonds provide the strong adhesion characteristics for which organofunctional silanes are well known.5 With proper surface preparation and material selection, organofunctional silane-based coatings can form siloxane bonds to many sites on the inorganic surface, forming an organofunctional siloxane network in the process (Figure 1). This organofunctional silane film can passivate the surface of a metal substrate, providing a barrier to keep water and salts from coming in contact with the metal surface. Furthermore, the organofunctional groups can provide additional hydrophobicity and adhesion promotion of any subsequent organic topcoats that may be applied for further corrosion protection.
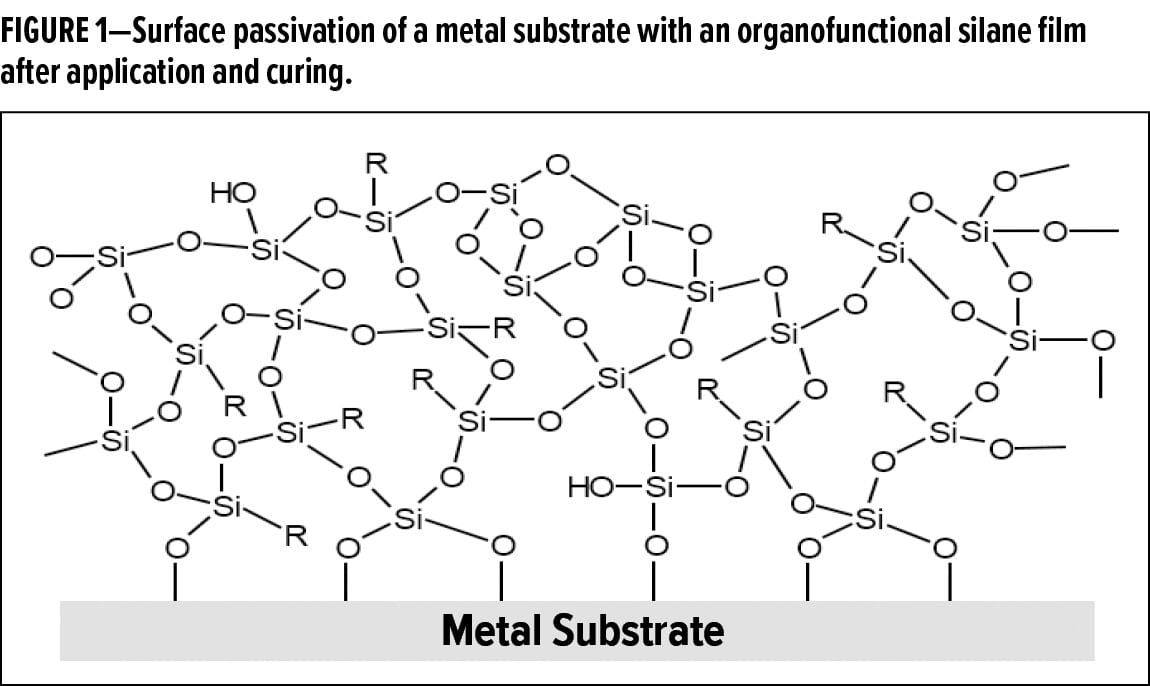
As previously mentioned, an elevated temperature curing procedure is typically required to drive off all the moisture in an organofunctional silane coating. This thermal curing procedure is not always feasible depending on the specific application or industry, and this has led to the exploration of alternative curing methods for organofunctional silane coatings. With the use of organofunctional bipodal silanes, the additional crosslinking density introduced into the system may alleviate the need for this elevated temperature curing procedure. This additional crosslinking density stems from the influx of alkoxy groups from the organofunctional bipodal silane. While it is possible for these additional alkoxy groups to undergo crosslinking at room temperature, the condensation of silanol groups is significantly accelerated at elevated temperatures.6 Additionally, the rate of crosslinking depends on several other factors, including pH, presence of solvents, and the concentration of silanes in the system.7 Although organofunctional trialkoxy silanes are commonly used in a wide variety of coating applications, organofunctional bipodal silanes, such as 1,2-bis(triethoxysilyl)ethane (Figure 2), can have six or more alkoxy groups. As these alkoxy groups undergo hydrolysis and condensation in the system, the additional siloxane bonds formed can accelerate the curing process of the system.8
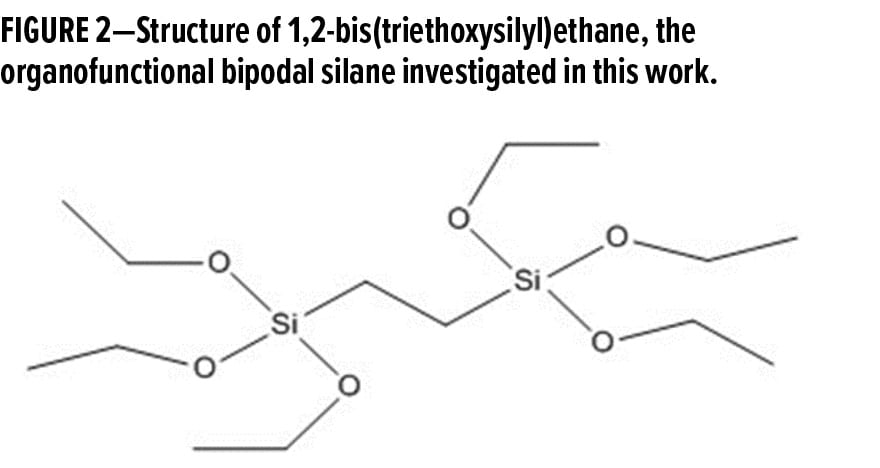
It is important to note that a two-carbon spacer links the six alkoxy groups on each side of 1,2-bis(triethoxysilyl)ethane. These alkyl chains are responsible for the hydrophobic nature of this organofunctional bipodal silane. For this reason, 1,2-bis(triethoxysilyl)ethane is commonly used in solvent-based systems, where the hydrophobic nature of this organofunctional bipodal silane does not interfere with its solubility in alcohol-based systems.9 Although it is rather difficult for hydrophobic organofunctional silanes to exhibit good stability in waterborne systems, optimizing the pH of the system to a slightly acidic value (pH 4–5) can maximize the hydrolysis rate and minimize the condensation rate of organofunctional bipodal silanes.10,11 This allows for improved solubility and hydrolytic stability of organofunctional bipodal silanes in waterborne systems.
The two waterborne systems that are presented in this work include a waterborne organofunctional silanol system with functionalized colloidal silica and a waterborne organofunctional silanol system without functionalized colloidal silica. Both these waterborne systems do not contain any volatile organic compounds, which is why they are commonly used as environmentally friendly alternatives to harmful corrosion resistance technology. The waterborne organofunctional silanol system with functionalized colloidal silica can also be used as a transparent sol-gel topcoat, while the waterborne organofunctional silanol system without functionalized colloidal silica can be used as a surface modifier for organic materials or as an adhesion-promoting additive into waterborne polymer systems. While these waterborne systems provide excellent corrosion resistance on their own, organofunctional bipodal silanes will be explored as performance-enhancing additives to these waterborne systems, in hopes of better understanding how to improve this new technology.
EXPERIMENTAL METHODS
Materials
1,2-Bis(triethoxy)silylethane (Dynasylan® * BTSE), waterborne organofunctional silanol system with functionalized colloidal silica (Dynasylan® SIVO 110), and waterborne organofunctional silanol system without functionalized colloidal silica (Dynasylan® HYDROSIL 2926) are available from Evonik Industries AG. Sodium hydroxide (99.99% pure) and ethyl alcohol (99.5% pure) were purchased from Sigma Aldrich. Bulk Kleen® * 737G (a proprietary alkaline powder cleaner) was purchased from Bulk Chemicals. Deionized (DI) water was obtained with a water purification system (WaterPro® † Plus) originally purchased from LabConco Corporation. Aluminum 6061T6® ‡ substrates were purchased from ACT Test Panels, LLC.
Formulation Preparation
The waterborne coatings evaluated in this article were formulated in 150 mL glass beakers (see Table 1 for ingredient breakdown) and allowed to mix for 96 h before use. This extensive mixing time is preferred to allow adequate time for the silane molecules in the formulation to hydrolyze and condense in the presence of water. After enough time, the condensation of silanol groups in the formulations will start to have a considerable impact on the viscosity of the coating, eventually leading to decreased film formation properties. This condensation rate is particularly low at pH 4–5 for the waterborne coatings evaluated in this article, allowing for approximately three weeks of sufficient stability before the onset of minor visual changes to the solutions. These visual changes could include precipitation, haziness, and increases in the viscosity of the system while mixing.
1,2-Bis(triethoxysilyl)ethane is 100% active solids, while the waterborne organofunctional silanol system with functionalized colloidal silica is 36% active solids, and the waterborne organofunctional silanol system without functionalized colloidal silica is 30% active solids. The final wt% solids of the waterborne coating formulations in this article were chosen to obtain transparent films that do not cause any negative optical characteristics to the metal surfaces. It is also important to note that the optimal silane concentration in a waterborne protective coating directly depends on the surface roughness of the metal substrate.12
Cleaning and Application Procedures
Metal Surface Cleaning Procedure
Before applying the waterborne formulations described above, it is crucial that the metal substrates are properly cleaned for optimal surface wetting properties. The metal substrates were first wiped with an ethyl alcohol-soaked paper towel. Following this solvent wipe, the metal substrates were dried with an air gun and placed in an alkaline washing solution (prepared by adding 15 g of Bulk Kleen® 737G to one liter of DI water and stirring for several hours before use) for 3 min at 140–150°F. The aluminum substrates were rinsed with DI water and dried with an air gun following the alkaline washing procedure.
Coating Application Procedure
After the metal substrates were properly cleaned and the waterborne coating formulations were allowed to fully hydrolyze, the coatings were applied via a dip coating procedure. The metal substrates were fully immersed in the waterborne silane formulations for 60 sec at room temperature, removed from the solution, and hung vertically in a fume hood for 10 min to allow the excess liquid to drip off the metal surface. Although some of the waterborne coating formulations were milky white, all the coatings evaluated in this article formed transparent films upon application.
Curing Procedure
After being allowed to dry at room temperature for 10 min following the dip coating procedure, the coated metal substrates were either left in the fume hood at room temperature to dry for an additional 48 h, or placed in an oven for 30 min at either 80°C (formulation WB1) or 180°C (formulations WB2, WB3, WB4, and WB5). It is important to note that after curing, the dry-film thicknesses of the waterborne organofunctional silanol system with functionalized colloidal silica and the waterborne organofunctional silanol system without functionalized colloidal silica were less than 1 mm.
Testing Procedures
Contact Angle Measurement Procedure
Once the coatings were fully cured, a goniometer (Ramé-Hart, Inc.) was used to measure the contact angle of DI water on the coated substrates. Each measurement reported in this article is the average of 10 contact angle measurements to ensure the accuracy of this method. The standard deviation of each set of 10 contact angle measurements represents the statistical error reported in this data. It is important to note that although the metal substrates were slightly bent during the production process, all measurements were done on aluminum substrates of the same production batch and at the same locations on each substrate.
Neutral Salt Spray Testing Procedure
Before evaluating the coated metal substrates in a neutral salt spray test, wax (IGI 1334 paraffin wax supplied by Lone Star Candle Making Co.) was used to coat the edges of the metal substrates. Corrosion resistance was evaluated in a Q-Fog® § Cyclic Corrosion Tester (The Q-Panel Company) according to ASTM B117.
Alkaline Resistance Testing Procedure
A solution containing 10% sodium hydroxide (NaOH) and 90% DI water was prepared by stirring at room temperature until all the NaOH pellets were fully dissolved. After properly applying and curing the waterborne coating formulations on the aluminum substrates, the panels were immersed in the alkaline solution for 10 min at room temperature. The panels were then removed, rinsed with DI water, observed visually, and then placed in a neutral salt spray test as described above.
Electrochemical Impedance Spectroscopy (EIS) Testing Procedure
EIS testing was performed by Matergenics, Inc. Gamry PCI4/750™* potentiostats were used to record the impedance spectra at frequencies of 0.1–100,000 cycles/sec. The coated metal panels were immersed in an aqueous conductive 3.5% NaCl solution during testing. To achieve a relatively stable open circuit potential for EIS measurements, the coated metal panels were immersed in the conductive 3.5% NaCl solution for 20 min before collecting impedance data. All measurements were performed in a grounded Faraday cage at room temperature.
RESULTS AND DISCUSSION
Surface Contact Angle Analysis
As mentioned previously, incorporation of an organofunctional bipodal silane into a waterborne system is expected to increase the hydrophobicity, surface passivation, and crosslinking density of the system, leading to improved corrosion resistance performance. In particular, 1,2-bis(triethoxysilyl)ethane was chosen for investigation in waterborne systems because of the two-carbon spacer group between the silicon functional groups on either side of the organofunctional bipodal silane. This two-carbon alkyl chain not only provides significant hydrophobicity, but is also a short enough chain to allow for sufficient solubility in waterborne systems.
While there are many ways of characterizing the hydrophobicity of a metal coating, valuable insight can be gained by visually observing the behavior of water on the coated surface. Water tends to spread out when placed on an uncoated aluminum surface, indicating a hydrophilic surface. On an aluminum surface that has been coated with a waterborne organofunctional bipodal silane coating, the water tends to bead up (Figure 3).
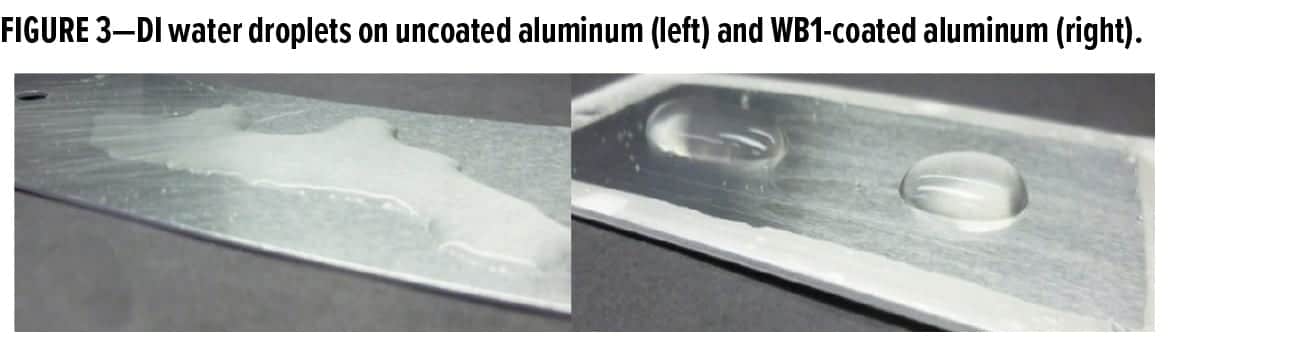
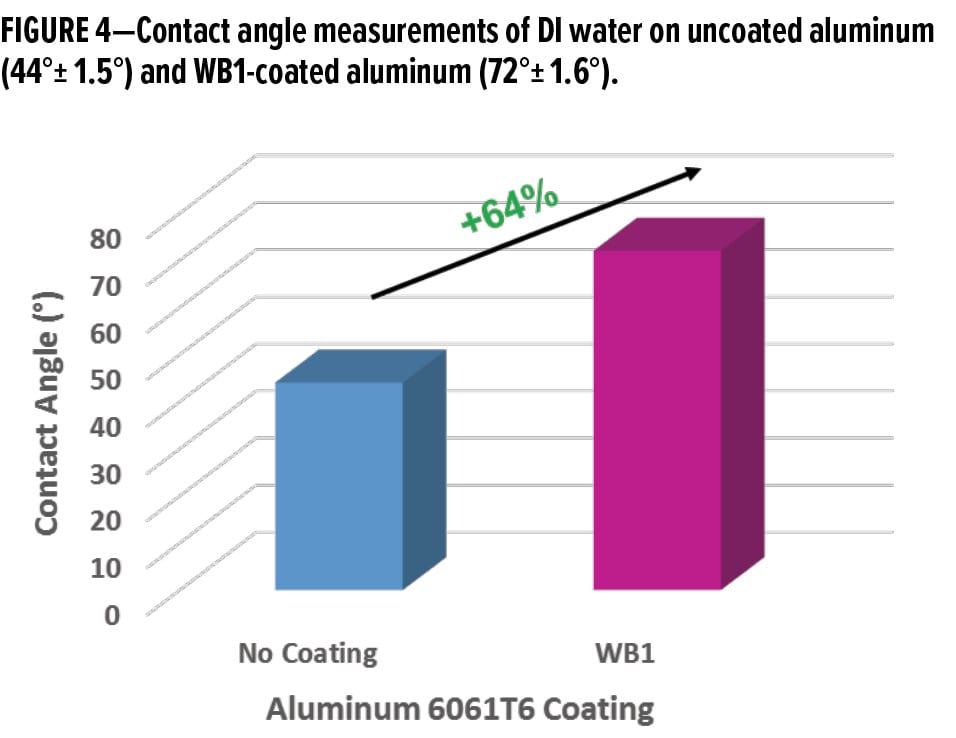
While visually observing the behavior of water on a metal surface is typically a good indication of whether the metal surface is hydrophilic or hydrophobic, contact angle measurements of water droplets on a metal surface can better quantify this behavior. The average contact angle of a DI water droplet on uncoated aluminum is 44° ± 1.5°, while the average contact angle of a DI water droplet on WB1-coated aluminum was 72° ± 1.6° (Figure 4). Thus, coating the aluminum surface with a waterborne organofunctional bipodal silane coating increased the contact angle of DI water by ~64%. Because a surface is typically considered hydrophobic when the contact angle of water on the surface is > 90°, it can be said that the this waterborne organofunctional bipodal silane system made the surface less hydrophilic.
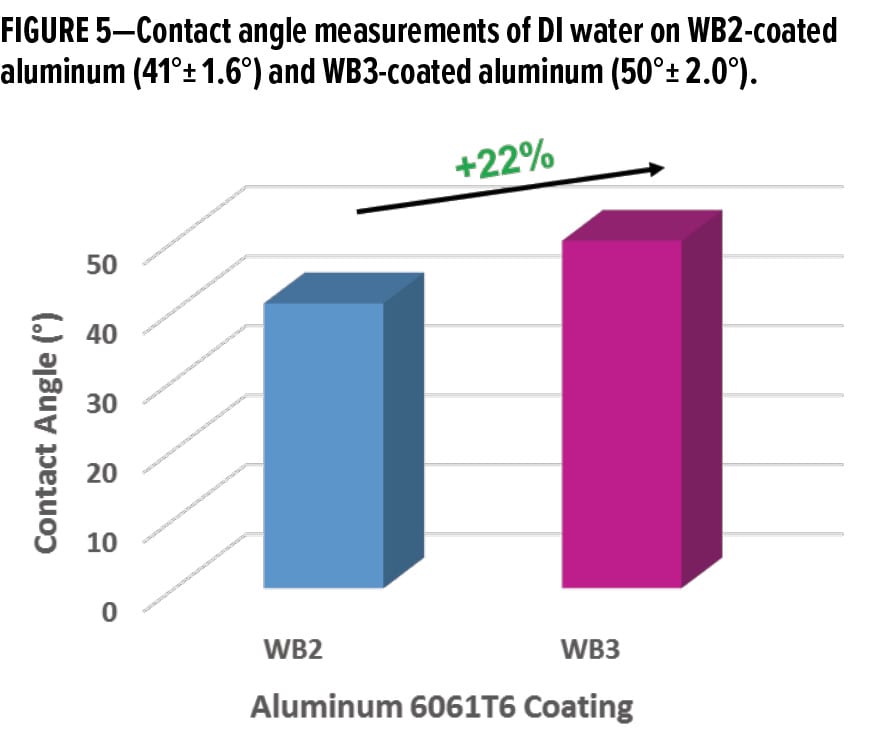
While the average contact angle of a DI water droplet on WB2-coated aluminum was 41°± 1.6°, adding an organofunctional bipodal silane into the system increased the average contact angle of a DI water droplet to 50° ± 2.0° (Figure 5). This ~22% increase in the average contact angle of DI water on WB3-coated aluminum indicates a significantly less hydrophilic surface than the WB2-coated aluminum.
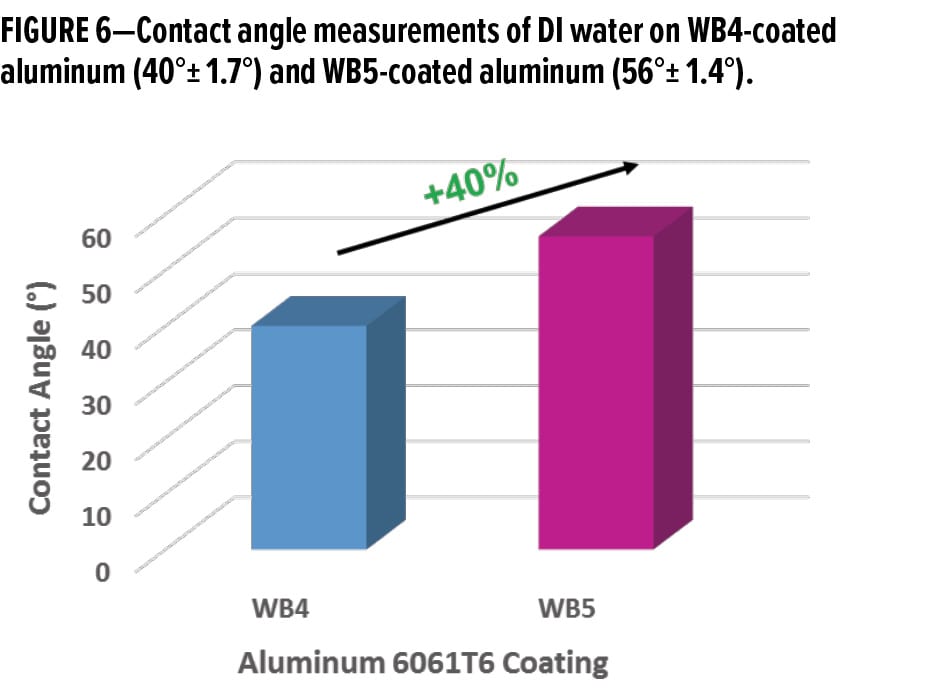
A ~40% increase in the average contact angle of DI water was observed when an organofunctional bipodal silane was incorporated into the waterborne organofunctional silanol system without functionalized colloidal silica. This indicates a significant decrease in the hydrophilic characteristics of the aluminum surface. The average contact angle of a DI water droplet on WB4-coated aluminum was 40° ± 1.7°, while the average contact angle of a DI water droplet on WB5-coated aluminum was 56°± 1.4° (Figure 6).
As confirmed by contact angle analysis, the incorporation of an organofunctional bipodal silane into the waterborne coatings described above decreased the hydrophilicity of the systems. This reduction in hydrophilicity should help prevent water droplets in the surrounding environment from properly wetting and coming into contact with the metal surface. While it can be speculated that less contact between water and the metal surface leads to lower corrosion rates, further corrosion testing was necessary to confirm this hypothesis.
Neutral Salt Spray Testing
While it has been debated that cyclic prohesion testing provides a more accurate prediction of corrosion resistance in real life conditions, neutral salt spray testing has been an industry standard for evaluating corrosion resistance for decades.13 However, it is important to note that the corrosion mechanism occurring in a salt spray chamber is fundamentally different than corrosion mechanisms in the real world. In a controlled salt spray chamber, the humidity, temperature, and sprayed salt solution is precisely controlled and monitored in a closed environment. In the field, the humidity, temperature, and exposure to varying weather patterns introduce many more variables when evaluating the performance of a corrosion protection coating. Although a coatings’ ability to prevent corrosion in a controlled humid and salty environment is often a good indicator of corrosion resistance performance out in the field, this will have to be confirmed with outdoor weatherability studies during future testing.
After coating aluminum substrates with the waterborne sol-gel silane coatings, three different curing procedures were employed. One set of panels was left to dry at room temperature for 72 h, while the other two sets of panels were baked in the oven for 30 min at either 80°C or 180°C. Without any heat to drive off the moisture in the waterborne sol-gel silane coatings cured at room temperature, poor adhesion and corrosion resistance was expected due to the low condensation rate between silanol groups in the coating, which inhibits the surface passivation of the metal surface. Additionally, such low condensation rate typically results in insufficient adhesion between the coating and the metal surface.
By incorporating an organofunctional bipodal silane into the waterborne organofunctional silanol system with functionalized colloidal silica, the influx of silanol groups in the system should increase the rate of condensation between silanol groups in the coating, leading to better surface passivation of the metal surface. Furthermore, these additional silanol groups should also increase the rate of condensation between the silanol groups in the coating and the hydroxyl groups on the metal surface, resulting in better adhesion to the metal, and better corrosion resistance.
These hypotheses can be supported by the superior corrosion resistance demonstrated by the waterborne organofunctional silanol system with functionalized colloidal silica and organofunctional bipodal silanes in the system (Figures 7 and 8).


This holds true for the coatings cured at room temperature for 72 h and elevated temperatures as well. In particular, no corrosion or defects were found on the WB3-coated aluminum substrate cured at 180°C after 250 h in a neutral salt spray test. It is important to note that no additional organic topcoats were applied on the waterborne coatings for this neutral salt spray test data.
Just as the presence of an organofunctional bipodal silane in the waterborne organofunctional silanol system with functionalized colloidal silica improved the corrosion resistance of the coating, similar performance improvements were observed in the waterborne organofunctional silanol coatings without functionalized colloidal silica as well. Although these systems are not identical, the addition of an organofunctional bipodal silane into the waterborne organofunctional silanol system without functionalized colloidal silica should also increase the crosslinking density, hydrophobicity, and surface passivation of the system. These hypotheses are supported by the superior performance of the waterborne organofunctional silanol coatings without functionalized colloidal silica and organofunctional bipodal silane additions in neutral salt spray testing (Figures 9 and 10).


It is important to note that although only a few hundred hours of neutral salt spray performance are shown here, the trends regarding better corrosion resistance of systems with organofunctional bipodal silane additions were consistent for over 1000 h of neutral salt spray testing. Additional layers of organic topcoats would also significantly improve the corrosion resistance of the aluminum substrates over time. While this is a promising glimpse of performance improvements achievable with organofunctional bipodal silane additives, other corrosion-related testing procedures are crucial in determining the full scope of the corrosion resistance performance for these systems.
Alkaline Resistance Testing
When it comes to the automotive industry, the alkaline resistance of a coating is often just as important as the corrosion resistance performance. This is due to the wide variety of automotive coatings that are subject to alkaline detergents, which can be found in most car wash fluids on the market nowadays. For exterior automotive coating applications, sealants are often applied on aluminum surfaces to provide corrosion resistance and alkaline resistance.14 As previously hypothesized, the addition of an organofunctional bipodal silane into a waterborne organofunctional silanol coating with functionalized colloidal silica increases the crosslinking density, hydrophobicity, and surface passivation of the system. While it has already been demonstrated that these additional characteristics from the organofunctional bipodal silane boost the corrosion resistance of the system, the alkaline resistance of the system can also be similarly improved.
It is important to note that the alkaline solution (pH 14) used in this test produces a much harsher environment than the neutral salt spray test environment. Within minutes of immersion, all the waterborne organofunctional silanol systems with functionalized colloidal silica and no organofunctional bipodal silane additives began to bubble violently and blackened the alkaline solution (Figure 11).

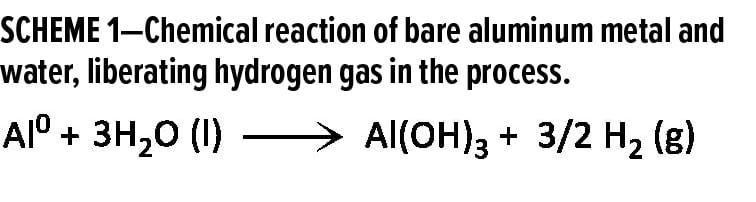
These bubbles are likely to be hydrogen gas, which is generated when bare reactive aluminum comes into direct contact with water (Scheme 1). This reaction occurs when the waterborne coating and protective aluminum oxide surface on the bare aluminum metal are removed by the high concentration of –OH groups in the alkaline solution.15
The addition of an organofunctional bipodal silane into the waterborne organofunctional silanol system with functionalized colloidal silica significantly reduced the amount of observable bubbling on the surface of the aluminum substrates. This can be explained by the lack of bare reactive aluminum metal exposed to the alkaline media due to the additional crosslinking and surface passivation from the organofunctional bipodal silanes in the coating. Upon removing the aluminum substrates from the alkaline media after 10 min of immersion, the waterborne organofunctional silanol coating with functionalized colloidal silica and no organofunctional bipodal silane additives was completely dissolved (Figure 12), and the aluminum surface was observably deformed. On the other hand, the waterborne organofunctional silanol system with functionalized colloidal silica and organofunctional bipodal silane additives only showed minor surface defects after the alkaline resistance test (Figure 13).


After prolonged exposure to alkaline media, the ability for a coating to continue exhibiting sufficient corrosion resistance is crucial in the automotive industry. For this reason, the coated aluminum substrates were rinsed with DI water following the alkaline resistance tests, then immediately placed in a neutral salt spray for 100 h, and evaluated for corrosion resistance. The aluminum substrate coated with the waterborne organofunctional silanol system with functionalized colloidal silica and no organofunctional bipodal silane additives showed significant change in appearance after the neutral salt spray testing. These changes included a significant discoloration of the entire aluminum surface, indicating rust build-up due to the lacking presence of the corrosion protection coating that was eradicated by the alkaline media. On the other hand, the aluminum substrate coated with the waterborne organofunctional silanol system with functionalized colloidal silica and organofunctional bipodal silane additives in the system exhibited much better corrosion resistance (Figure 14). Although ~50% of the aluminum surface showed signs of minor corrosion, no significant discoloration due to rust build-up was observed.
While this corrosion resistance of the organofunctional bipodal silane-containing system was not perfect, higher loading levels of the organofunctional bipodal silane could further enhance this post-alkaline test corrosion resistance performance.

Electrochemical Impedance Spectroscopy
While contact angle measurements have shown the impact of organofunctional bipodal silanes on the hydrophobicity of waterborne coatings, and salt spray tests have demonstrated the corrosion resistance performance of the coatings, EIS can also offer valuable insight into the barrier properties of these organic coatings on metal substrates. EIS is a useful characterization technique for analyzing coated metal substrates because the deterioration of a metal coating in the presence of an electrolyte solution can be monitored in real time.16 By simultaneously measuring the resistance and capacitance of an organic coating on a metal substrate, the impedance of the system can be calculated. As organic coatings are nonconductive in nature, they typically exhibit very high impedances in the presence of an electrolyte. The better the barrier properties of an organic coating, the lesser is the amount of electrolyte solution that is directly exposed to the metal substrate, which subsequently results in high impedance measurements for the system. While an organic coating may exhibit high impedances immediately after the coated metal substrate is immersed in the electrolyte solution, the impedance of the system will drop as the electrolyte continuously penetrates the organic coating and reaches the underlying metal substrate.17 Even though this initial corrosion of the metal substrate may only be occurring over microscopic surface areas, EIS can detect these small changes in impedance when no visible corrosion has appeared on the surface of the coated metal substrates.18 Impedance measurements are typically carried out across a broad range of frequencies, and are represented in a Bode plot (Figure 15).
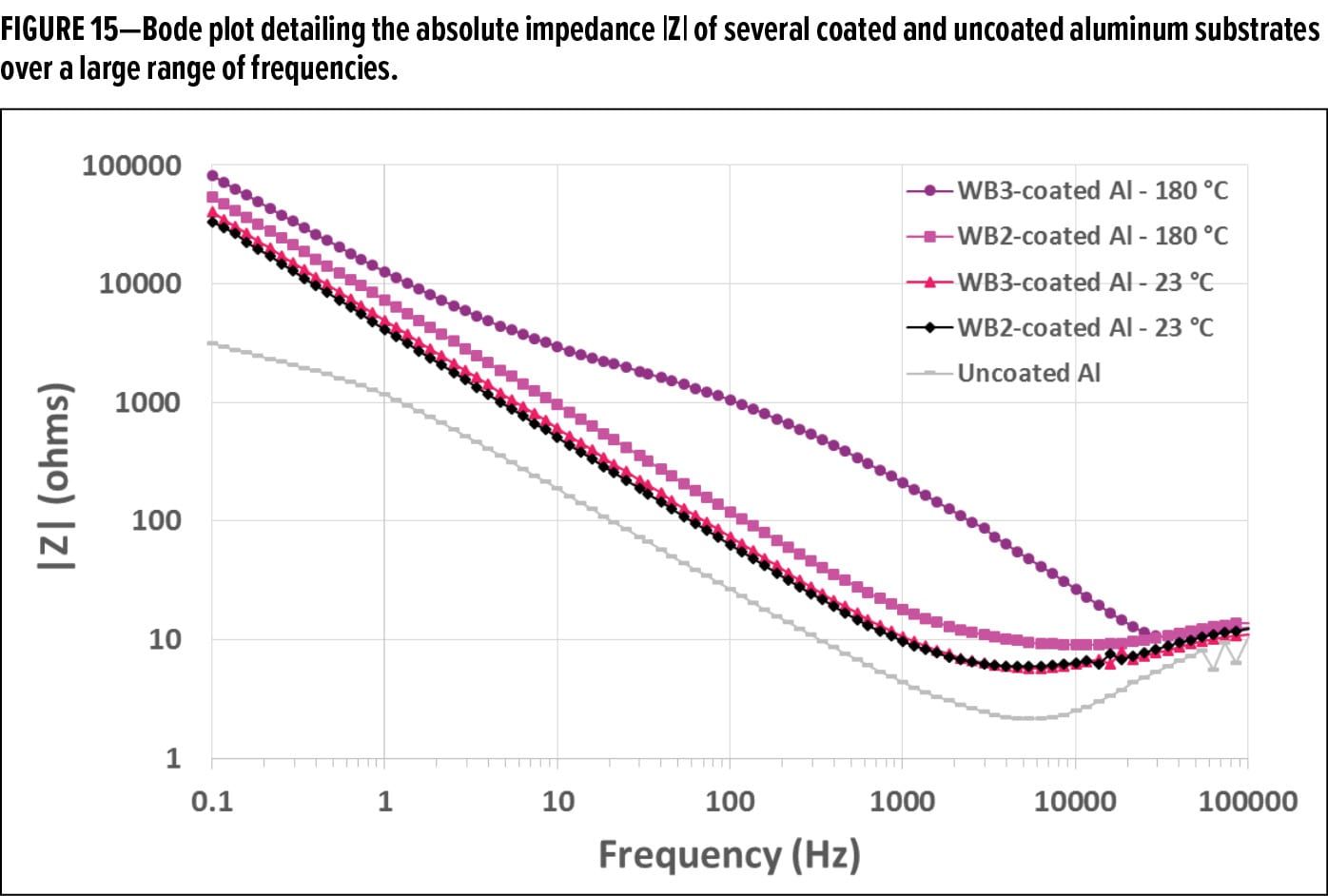
One of the most evident observations of EIS measurements is the difference in the magnitude of the impedance for each corrosion protection system. Comparing the impedance magnitude (in ohms, Ω) of these systems at 0.1 Hz is a quick, well-established prediction of how well a corrosion-protection coating will perform as a barrier over time.19 At 0.1 Hz, the impedance of the thermally cured waterborne organofunctional silanol coating with functionalized colloidal silica and an organofunctional bipodal silane was ~82 kΩ (82,000 ohms). Without the organofunctional bipodal silane in this thermally cured waterborne organofunctional silanol coating, the impedance at 0.1 Hz was ~54 kΩ, a ~34% reduction in impedance. Addition of an organofunctional bipodal silane into the room temperature cured waterborne organofunctional silanol coatings with colloidal silica improved the impedance of the system from ~33 kΩ to 40 kΩ at 0.1 Hz, a ~17% improvement in impedance. It is important to note that the dry-film thicknesses for all these pretreatment systems were approximately the same (less than 1 µm), thus not contributing to the difference in impedance observed in these EIS measurements.
The lowest impedance across all the frequency measurements was observed with the uncoated aluminum substrate. Without a coating to act as a barrier to the water and salts in the surrounding environment, corrosion can form rapidly and degrades the surface over time.
With the application of a waterborne organofunctional silanol coating with functionalized colloidal silica on the aluminum surface, the barrier effect is increased, subsequently increasing the impedance of the system over a wide range of frequencies.
Addition of an organofunctional bipodal silane into the waterborne organofunctional silanol coating with functionalized colloidal silica significantly increased the impedance of the system over the range of 0.1 Hz to 20,000 Hz. Additionally, coatings cured at 180°C exhibited significantly higher impedance measurements than the coatings cured at room temperature. This can best be explained by the additional crosslinking introduced into the waterborne coating by both the organofunctional bipodal silane and the heat from the thermal curing procedure. The organofunctional bipodal silane increases the crosslinking density and surface passivation with additional alkoxy groups being introduced into the system, while the thermal curing procedure accelerates the formation of siloxane bonds between the aluminum substrate and the coating through driving condensation. Both the organofunctional bipodal silane additives and elevated temperature cure increase the impedance of the system by making the coating a more efficient barrier to water and salts in the surrounding environment. While contact angle measurements gave insight into the hydrophobicity of these waterborne systems, and salt spray testing gave observable corrosion resistance evidence, these EIS measurements further confirmed the hypothesis that organofunctional bipodal silanes can improve the corrosion resistance of waterborne systems by increasing the surface passivation of the coatings.
CONCLUSION
As waterborne silane technology continues to gain interest as an environmentally friendly alternative to well-established corrosion-protection technology, investigations into performance-enhancing additives are crucial for supporting this market growth. It can be concluded that organofunctional bipodal silanes are viable performance-enhancing additives in waterborne corrosion-protection systems due to the increase in hydrophobicity, crosslinking density, and surface passivation that these materials can provide. In particular, the waterborne organofunctional silanol system with colloidal silica and organofunctional bipodal silane additives exhibited the best corrosion resistance in neutral salt spray testing, the best alkaline resistance in alkaline testing, and the highest impedance during EIS testing. However, it is important to note that adding in an organofunctional bipodal silane into a room temperature cured waterborne organofunctional silanol coating did not outperform a thermally cured waterborne organofunctional silanol coating without organofunctional bipodal silane additives. While contact angle measurements, salt spray testing, alkaline resistance testing, and EIS data support this claim of increased corrosion resistance with the use of organofunctional bipodal silane additives, further research is necessary to understand the complete scope of waterborne silane technology and its interactions with organofunctional bipodal silanes. Additional experimentation, including outdoor weatherability testing in real life conditions, is underway to better understand this technology in hopes of further improving the performance, affordability, and reliability of waterborne silane coatings for corrosion resistance applications.
Presented at the 46th International Waterborne, High-Solids, and Powder Coatings Symposium, February 24–March 1, 2019, in New Orleans, LA.
*Dynasylan® is a registered trademark of Evonik Degussa GmbH.
*Bulk Kleen® is a registered trademark of Bulk Chemicals Inc.
† WaterPro® Plus is a registered trademark of Bulk Chemicals Inc.
‡ Aluminum 6061T6® is a registered trademark of ACT Test Panels, LLC.
§Q-Fog® is a registered trademark of Q-Lab Corporation.
*Gamry PCI4/750™ is a trademark of Gamry Instruments.
References
1. Kendig, M., Jeanjaquet, S., Addison, R., and Waldrop, J., Surf. Coat. Technol., 140 (1), 58–66 (2001).
2. Fedrizzi, L., Deflorian, F., Rossi, S., Fambri, L., and Bonora, P., Prog. Org. Coat., 42 (1-2), 65–74 (2001).
3. Zhu, D. and van Ooij, W.J., Prog. Org. Coat., 49 (1), 42–53 (2004).
4. Cave, N. and Kinloch, A., Polym., 33 (6), 1162–1170 (1992).
5. Plueddemann, E., J. Adhesion, 2 (3), 184–201 (1970).
6. Monticelli, F., Toledano, M., Osorio, R., Ferrari, M., Dental Mater., 22 (2006).
7. Liu, Q., Ding, J., Chambers, D., Debnath, S., Wunder, S., and Baran, G., J. Biomed. Mater. Res., 57 (3), 384–393 (2002).
8. Song, J. and van Ooij, W.J., Adhes. Sci. Technol., 17, 2191–2221 (2003).
9. Zhendong, S., Yanning, Y., Qingpeng, L., Jianguo, L., and Chuanwei, Y., Adv. Mater. Res., 971–973, 135–138 (2014).
10. Salon, M.-C., Bayle, P.-A., Abdelmouleh, M., Boufi, S., and Belgacem, M., Coll. Surf. A: Physicochem. Engin. Aspects, 312 (2-3), 83–91 (2008).
11. Brinker, C.J., J. Non-Cryst. Solids, 100 (1-3), 31–50 (1988).
12. Zhao, H., Yu, M., Liu, J., and Li, S., J. Electrochem, Soc,, 162 (14), C718–C724 (2015).
13. Skerry, B., Alavi, A., and Lindgren, K., Coat. Technol., 60, 97 (1988).
14. Manavbasi, A., Bodily, K., Clarke, T., Johnson, K., and Estes, B., Met. Finish., 111 (6), 12–15 (2013).
15. Zhang, J., Klasky, M., and Letellier, B., J. Nucl. Mat., 384, 175–189 (2009).
16. Bierwagen, G., Tallman, D., Li, J., and He, L., Prog. Org. Coat., 46, 148 (2003).
17. Sharer, Z. and Sykes, J., Prog. Org. Coat., 74 (2), 405–409 (2012).
18. Chico, B., Galvan, J.C., de la Fuente, D., and Morcillo, M., Prog. Org. Coat., 60, 45–53 (2007).
19. Gray, L. and Appleman, B., J. Protect. Coat. & Linings, 66 (2003).
CoatingsTech | Vol. 16, No. 4 | April 2019
